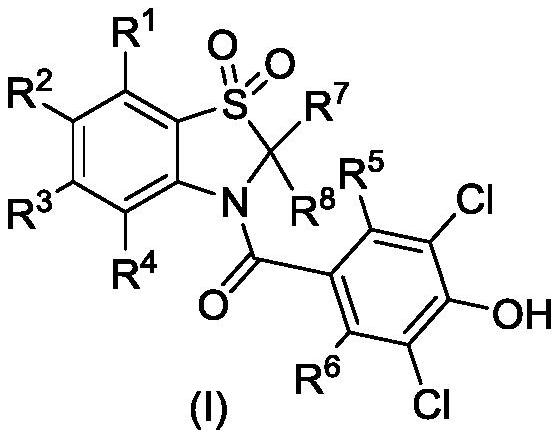
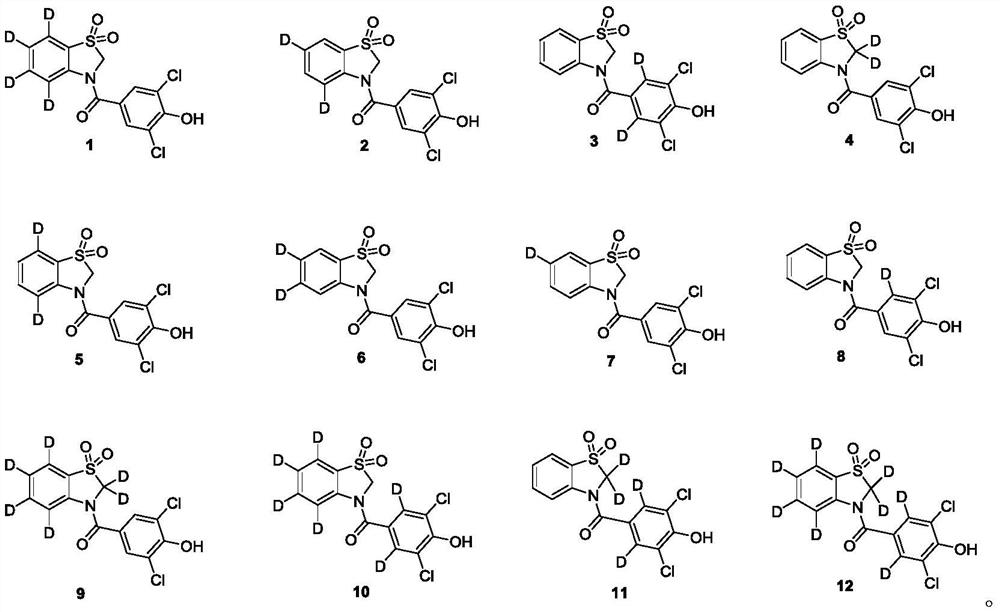
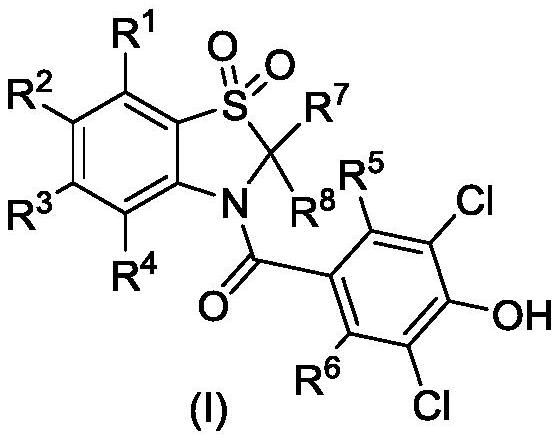
Compound for preventing, treating or alleviating hyperuricemia or gout and application thereof
A uric acid and composition technology, applied in the field of medicinal chemistry, can solve the problems of not being able to use for a long time, and the sales of Resinard is not high, and achieve the effect of long half-life, good stability, and strong URAT1 inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 compound 1
[0048] The reaction scheme is as follows:
[0049]
[0050] Specific steps are as follows:
[0051] Step 1: Synthesis of Intermediate 1-1
[0052] Dissolve tetrachloronitrobenzene S1 (13.05g, 50mmol) in dimethylsulfoxide (100mL), add sodium hydrosulfide (2.80g, 50mmol), and react at room temperature for 24h. The reaction solution was quenched with dilute hydrochloric acid (2M, 50 mL), added water (500 mL), extracted with ethyl acetate (3×500 mL), and the organic layer was dried over anhydrous magnesium sulfate, filtered and concentrated. The crude product was purified by column chromatography (silica gel, eluent was dichloromethane / methanol, 20 / 1 to 3 / 1 gradient elution) to obtain 6.98 g of yellow powder 1-1, yield 54%.
[0053] 1 H NMR (300MHz, CDCl 3 )δ5.12(s,1H),8.57(s,1H).
[0054] ESI-MS(m / z):257.2(M+H + )
[0055] Step 2: Synthesis of Intermediate 1-2
[0056] 1-1 (5.17g, 20mmol) was dissolved in ethanol (80...
Embodiment 2
[0077] The reaction scheme is as follows:
[0078]
[0079] Specific steps are as follows:
[0080] Step 1: Synthesis of Intermediate 2-1
[0081] 2,6-dichloro-4-aminophenol S3 (5.34g, 30mmol), deuterated hydrochloric acid solution (20%w / w in D 2 (2) (1.5 mL) was added into heavy water (30 mL), and refluxed for 72 h under the protection of argon. After cooling to room temperature, excess potassium carbonate was added to neutralize the reaction mixture. Extracted with ethyl acetate (3×100 mL), the organic layer was dried over anhydrous magnesium sulfate, filtered and concentrated, and purified by column chromatography (silica gel, eluent was dichloromethane / methanol, gradient 20 / 1 to 1 / 1 washing) to obtain 4.91 g of brown oil 2-1. Yield 91%, deuterated rate 96%.
[0082] 1 H NMR (300MHz, CDCl 3 )δ6.55 (undeuterated part, s, 0.08H), 6.16(s, 2H), 9.80(s, 1H).
[0083] ESI-MS(m / z):180.3(M+H + )
[0084] Step 2: Synthesis of Intermediate 2-2
[0085] Slowly add 2-1 (4...
Embodiment 3
[0097] The reaction scheme is as follows:
[0098]
[0099] Specific steps are as follows:
[0100] Step 1: Synthesis of Intermediate 4-1
[0101] Dilute paraformaldehyde-d2 (1.5 g, 50.0 mmol) with water (10 mL), add diisopropyl ether (10 mL) and S5 (626 mg, 5.0 mmol), and react overnight at room temperature. The organic layer was separated and the aqueous layer was extracted with diisopropyl ether (3 x 20 mL). The organic layers were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain 560 mg of brown oil 4-1 with a purity of 90% by LCMS and a yield of 80%, which was directly used in the next reaction without purification.
[0102] ESI-MS (m / z): 140.1 (M+H + )
[0103] Step 2: Synthesis of Intermediate 4-2
[0104] 3,5-Dichloro-4-hydroxybenzoic acid S2 (414mg, 2.00mmol) was dissolved in N,N-dimethylformamide (10mL), and N,N,N',N'-tetramethyl- O-(7-azabenzotriazol-1-yl)urea hexa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com