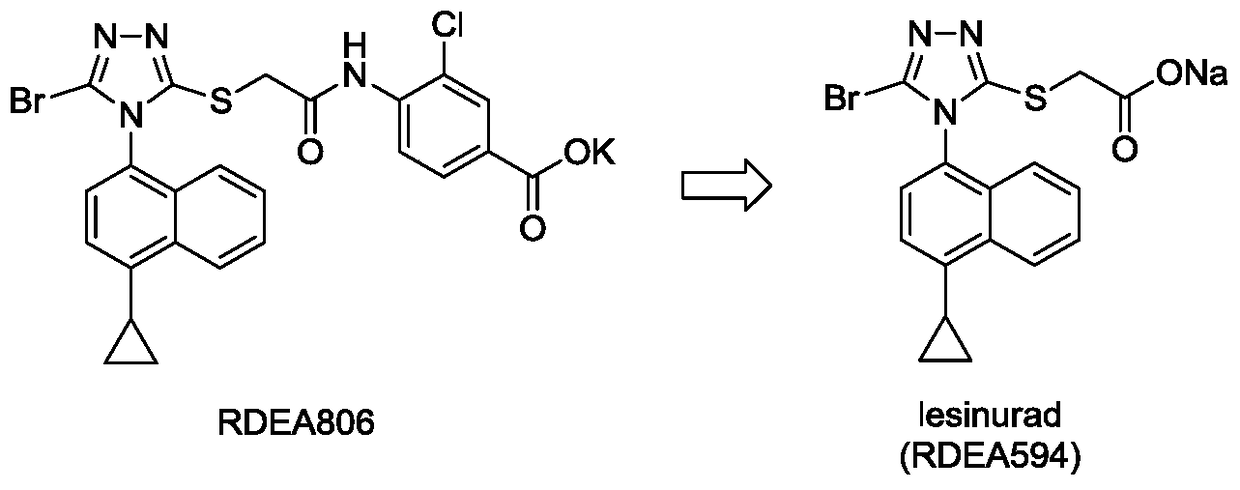
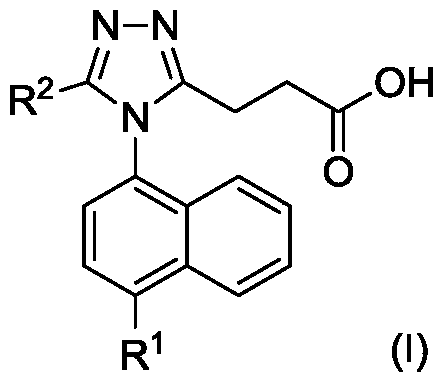
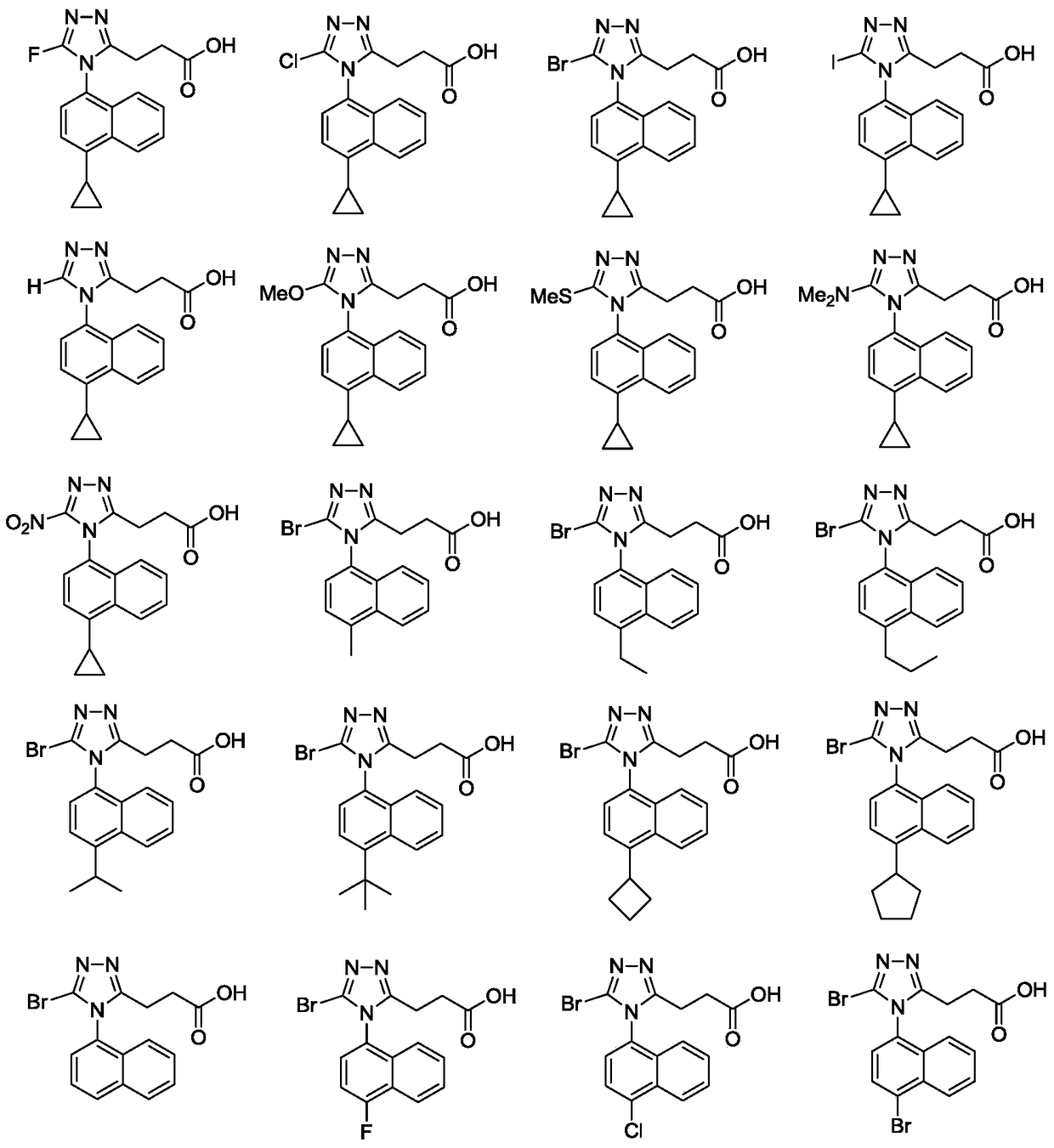
Triazole n-propionic acid urat1 inhibitor, preparation method and use thereof in the treatment of hyperuricemia and gout
A halogenated agent, XI-A technology, applied in the direction of bone diseases, drug combination, etc., can solve problems such as unsuitable for long-term treatment, serious side effects, patient compliance is not as good as oral preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The synthesis of embodiment 1 compound I-1
[0031]
[0032] Step 1. Synthesis of Compound VI-1
[0033] 4-Pentenylhydrazide II was synthesized according to literature method (Gilchrist, T.L.; et al. Synthesis, 1983, 153-154). 4-Pentenylhydrazide II (11.41g, 100mmol) and N,N-dimethylformamide dimethyl acetal III (11.92g, 100mmol) were dissolved in 230mL acetonitrile, heated and stirred at 50°C until TLC The reaction was found to be complete (usually around 0.5-1 hour).
[0034] After the reaction was completed, the reaction mixture was slightly cooled and concentrated to 1 / 3 of the original volume on a rotary evaporator, at this time a solution of IV was obtained. 4-cyclopropylnaphthylamine V-1 (18.32 g, 100 mmol) and 230 mL of glacial acetic acid were added thereto, and the reaction mixture was stirred overnight at 120° C. under nitrogen protection, and TLC inspection found that the reaction was complete.
[0035] After the reaction mixture was cooled, it was po...
Embodiment 2
[0054] The synthesis of embodiment 2 compound 1-2
[0055]
[0056] Step 1. Synthesis of Compound XI-A-2
[0057] Compound X-1 (0.96g, 3mmol) was dissolved in 10mL of acetonitrile, stirred at room temperature, and N-chlorosuccinimide (NCS, 0.48g, 3.6mmol) was added. After the addition was complete, the reaction mixture was stirred at room temperature until completion by TLC (typically 24 hours).
[0058] The reaction mixture was poured into 100mL ice water, stirred, and washed with 50mL×3CH 2 Cl 2 extraction. The combined and extracted organic phases were successively washed with Na 2 S 2 o 3 Solution, 100mL×5 saturated Na 2 CO 3 solution and 5% saline, anhydrous Na 2 SO 4 dry. The dried organic phase was evaporated to remove the solvent on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain the product XI-A-2 as a white solid, 0.82 g, with a yield of 77%. MS, m / z=356 ([M+H] + ).
[0059] Step 2. Synthesis of Compoun...
Embodiment 3
[0062] The synthesis of embodiment 3 compound 1-3
[0063]
[0064] Step 1. Synthesis of Compound XI-A-3
[0065] Compound X-1 (4.82g, 15mmol) was dissolved in 50mL of acetonitrile, stirred at room temperature, and N-iodosuccinimide (NIS, 4.05g, 18mmol) was added. After the addition was complete, the reaction mixture was stirred at room temperature until completion by TLC (typically 24 hours).
[0066] The reaction mixture was poured into 300mL ice water, stirred, and washed with 100mL×3CH 2 Cl 2 extraction. The combined and extracted organic phases were successively washed with Na 2 S 2 o 3 Solution, 100mL×5 saturated Na 2 CO 3 solution and 5% saline, anhydrous Na 2 SO 4 dry. The dried organic phase was evaporated to remove the solvent on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain the product XI-A-3, a white solid, 5.43 g, with a yield of 81%. MS, m / z=448 ([M+H] + ).
[0067] Step 2. Synthesis of Compound 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com