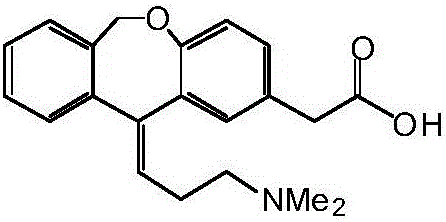
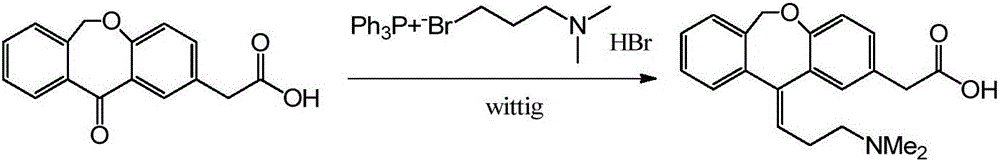
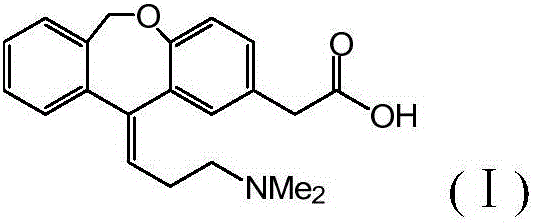
Preparation method of olopatadine
A technology of olopatadine and tetrahydrofuran, which is applied in the field of chemical drug preparation, can solve the problems of high cost, explosion, and long reaction time of olopatadine, and achieve the effects of shortening reaction time, reducing safety risks, and low environmental sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 (reference patent US20070232814)
[0044] Under the protection of nitrogen, add 2.4L of anhydrous-treated tetrahydrofuran into a 5L dry reaction kettle, stir and add 262g (6.56mol) of sodium hydride (60%), add hydrobromic acid-3-dimethylaminopropyl triphenyl bromide 816 g (1.6 mol) of phosphonium was stirred at room temperature for 1 hour, and the temperature was raised to 55-60° C. for 16 hours to react, turning blood red (gas was always generated). Cool to 20-25° C., add 216 g (0.8 mol) of isoket acid, and react at room temperature for 24 hours. TLC detection shows that the reaction is complete.
[0045] Cool to 10-15°C, add water and tetrahydrofuran solution (water: 100mL, THF: 400mL) dropwise, (severe heat release, a large amount of gas is generated), take about 4 hours, continue stirring for 30 minutes, filter, and rinse the filter cake with 500mLTHF . Combine the filtrates, stir and add concentrated hydrochloric acid to pH ~ 2, continue stirring for...
Embodiment 2
[0046] Embodiment 2 (adding N,N-dimethylformamide)
[0047] Under the protection of nitrogen, add 240mL of anhydrous-treated tetrahydrofuran and 8ml of N,N-dimethylformamide into a 500mL dry reaction kettle, cool in a water bath, stir and add 16.3g (0.41mol) of sodium hydride (60%), and add hydrogen Bromic acid-3-dimethylaminopropyltriphenylphosphonium bromide 81.6g (0.16mol), stirred at room temperature for 2 hours, heated to 55-60°C and reacted for 12 hours, turning blood red. Cool to 20-25° C., add 21.6 g (0.08 mol) of isoket acid, and react at room temperature for 24 hours. TLC detection shows that the reaction is not complete.
Embodiment 3
[0048] Embodiment 3 (adding dimethyl sulfoxide)
[0049] Under nitrogen protection, add 2.4 L of anhydrous-treated tetrahydrofuran and 80 ml of dimethyl sulfoxide into a 5 L dry reaction kettle, cool in a water bath, add 163 g (4.08 mol) of sodium hydride (60%) with stirring, and add 3-hydrobromic acid 816 g (1.6 mol) of dimethylaminopropyl triphenylphosphonium bromide was stirred at room temperature for 1.5 to 2 hours, then heated to 45°C for 1 hour. Cool to 20-25°C, add 216g (0.8mol) of isoket acid, react at room temperature for 10-12 hours, TLC detection shows that the reaction is complete.
[0050] Cool to 10-15°C, add water and tetrahydrofuran (water: 100mL, THF: 200mL) dropwise for about 1 hour, continue stirring for 30 minutes, filter, and rinse the filter cake with 500mL THF. Combine the filtrates, stir and add concentrated hydrochloric acid to pH ~ 2, continue stirring for 30 minutes, and filter. The filter cake was washed with 200 mL of tetrahydrofuran and dried in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com