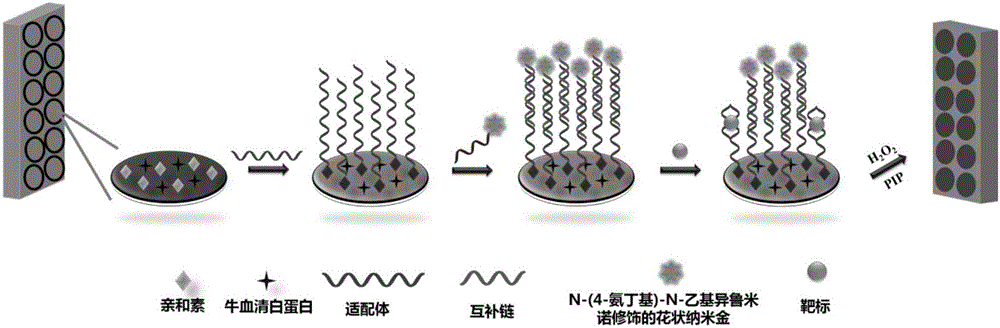
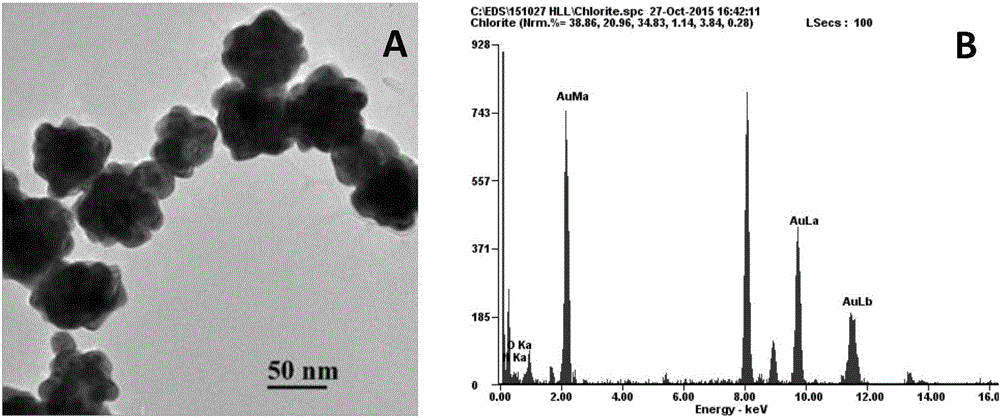
Method for simultaneously detecting oxytetracycline, tetracycline and kanamycin based on ABEI modified flower-shaped nanogold
A kanamycin and nano-gold technology, applied in the fields of nanomaterials and analytical chemistry, can solve the problems of increasing markers or labeling signals, increasing the difficulty of detection methods, restricting target types, etc., to improve stability and accuracy, and to facilitate Chemical modification, the effect of avoiding errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Detection of oxytetracycline, tetracycline and kanamycin in actual milk samples. Test sample pretreatment: first, add different concentrations of oxytetracycline, tetracycline, and kanamycin to milk samples, and dilute 40 times with ELISA treatment solution. Three different brands of milk were purchased from a local supermarket, and the content of ochratoxin A was determined by using the method of the present invention and the enzyme-linked immunosorbent method, and the results are shown in Table 1. The detection results of the two methods were consistent with no significant difference.
[0024]Table 1: The actual sample detection of milk, the method of the present invention is compared with the ELISA method
[0025]
[0026] Note: ND is not detected
Embodiment 2
[0027] Example 2: Detection of Oxytetracycline, Tetracycline and Kanamycin in Milk Actual Samples and the Experimental Sample Pretreatment of Standard Addition Recovery Rate is the same as in Example 1.
[0028] With the 3 groups of oxytetracycline that embodiment 1 obtains, tetracycline and kanamycin concentration data are background values, add three kinds of antibiotic standard substances of different concentrations wherein respectively, utilize the method of the present invention to detect again the antibiotic added wherein content to obtain the detection value. Recovery %=(detection value-background value) / addition amount X100%. From the data in Table 2, it can be seen that the recovery rate is 96.04% to 102.66%, indicating that the present invention is stable, sensitive and accurate, and is applicable to the detection of oxytetracycline, tetracycline and kanamycin in actual milk samples.
[0029] Table 2: Detection of oxytetracycline, tetracycline and kanamycin in actua...
PUM
| Property | Measurement | Unit |
|---|---|---|
| linear range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com