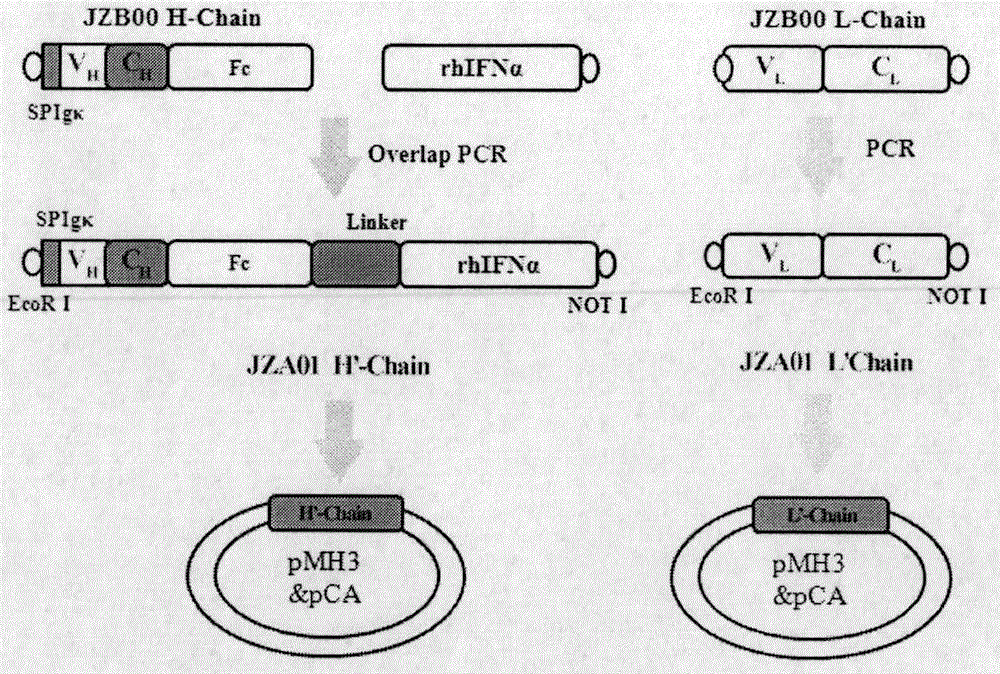
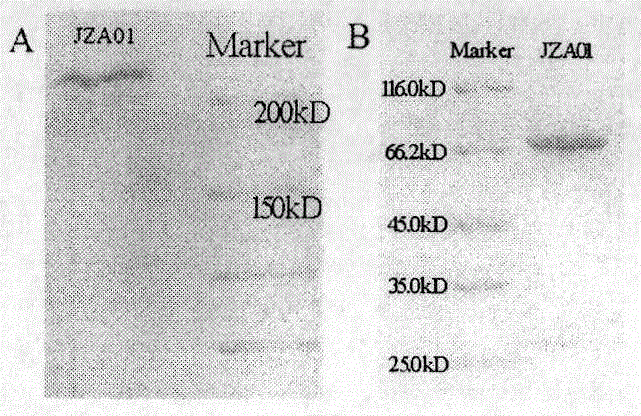
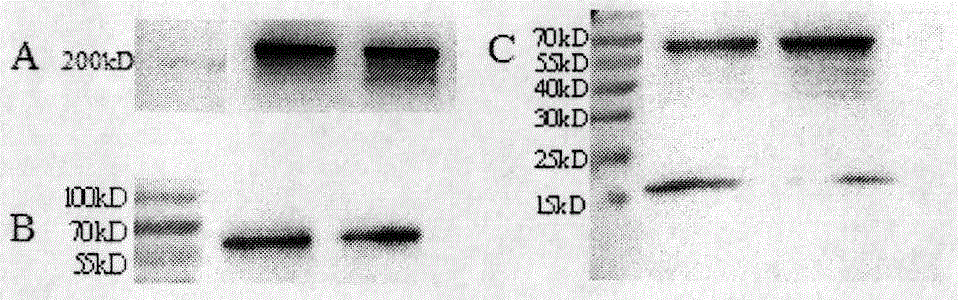
Preparation and application of cytokine fusion antibody
A technology of fusing antibodies and growth factor receptors, applied in the field of bioengineering, can solve the problems of strong systemic toxic and side effects, inability to reduce systemic toxic and side effects well, and short interferon half-life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
[0050] Example 4 Flow cytometric binding of anti-VEGFR2 fusion antibody to HUVEC cells
[0051] Digest HUVEC cells and divide into 1 x 10 6 Each group was centrifuged at 1500rpm at 4°C for 5min, washed three times with PBS+2%, and reacted with 100nM JZA01, 100nM JZB00 and skimmed milk solution at 4°C for 1h. Centrifuge at 1500 rpm at 4°C for 5 minutes, wash with PBS+2% FBS three times, and react with Donkey Anti-Human IgG (H+L)-FITC antibody (Life Sciences) at 4°C for 1 hour. Centrifuge at 1500rpm at 4°C for 5min, wash with PBS+2% for three times, resuspend the cells with PBS after the last centrifugation, and detect by flow cytometry.
Embodiment 5
[0052] Example 5 The growth inhibitory effect of anti-VEGFR2 fusion antibody on HUVEC and some tumor cells
[0053] Cells were prepared as 3 x 10 4 cells / ml cell suspension, inoculate 96-well cell culture plate, 100μl / well, at 37℃5%CO 2 Cultivate in the incubator for 24h. The drug was formulated with 2% fetal bovine serum medium, and there were three groups of JZA01, JZB00 and IFNα. Each group was set with 8 concentrations of 2.5nM, 5nM, 10nM, 20nM, 40nM, 80nM, 160nM, 320nM (0.01nM, 0.1nM, 1nM, 10nM, 100nM, 200nM total 6 concentrations). Discard the supernatant of the cultured cells, add drugs according to grouping, 100 μL per well, set up three parallel wells for each drug concentration in each group, continue to culture for 72 hours, add 11 μl of MTT to each well, continue to culture at 37 ° C for 4 hours, carefully pour off the upper 150 μl DMSO was added to each well, and the light absorption value (OD value) was measured at a wavelength of 570 nm / 630 nm on a microplate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com