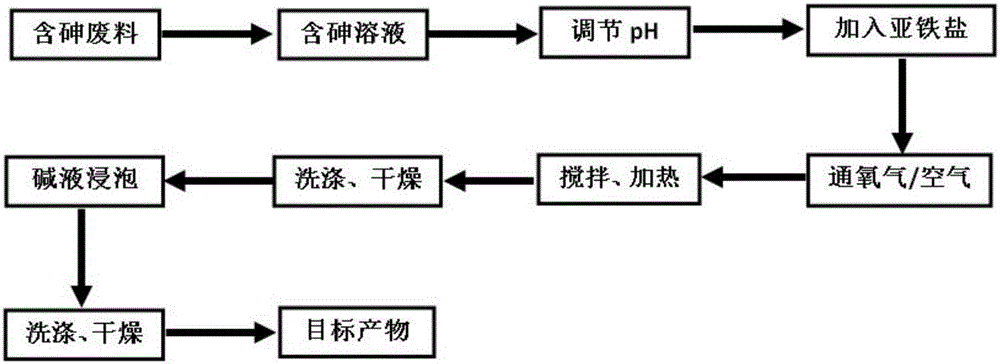
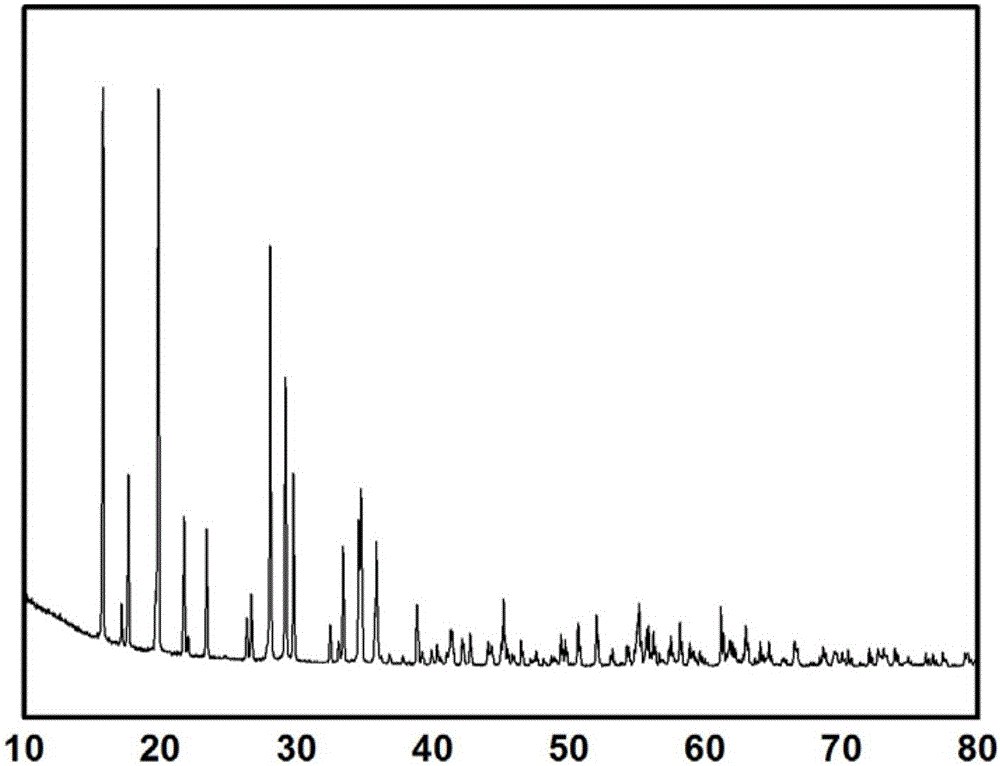
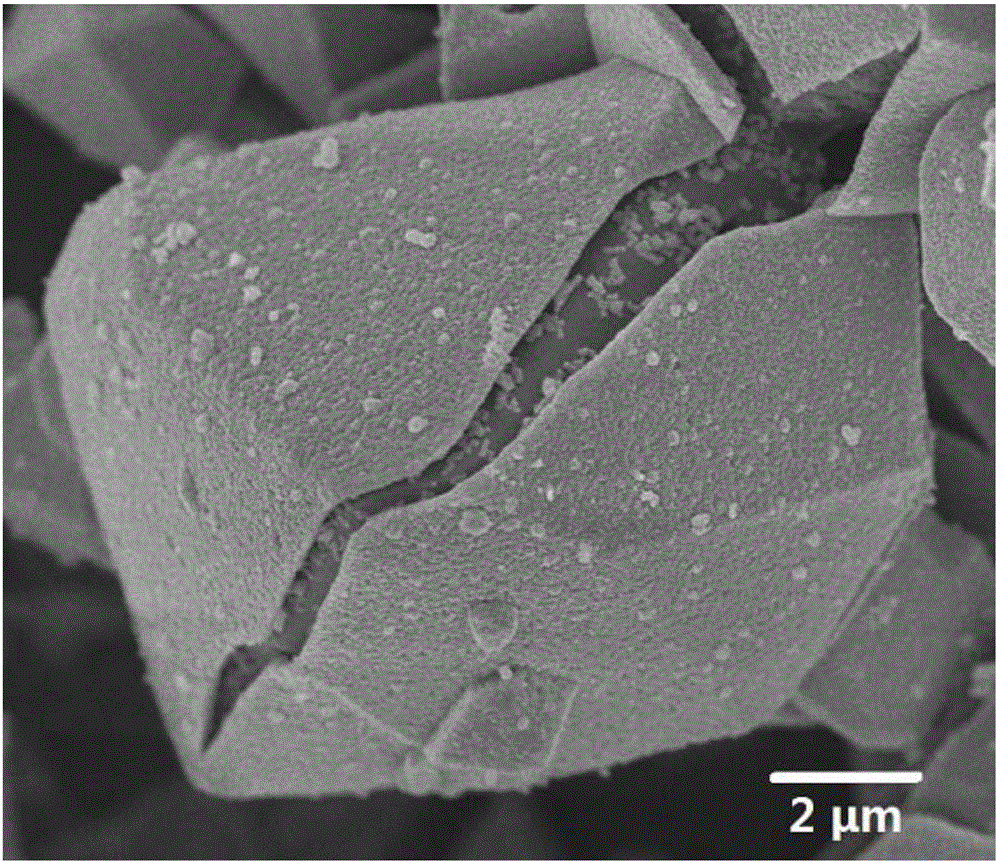
Method for fixing arsenic by preparing ferric arsenate/goethite material of core-shell structure by means of arsenic-containing solution
A core-shell structure, iron arsenate technology, applied in the field of environmental treatment, can solve the problems of poor arsenic fixation effect and high cost of arsenic fixation, and achieves the effects of simple steps, low price, and favorable popularization and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Prepare 1 liter of 30 g / L arsenic-containing solution according to the above method, and adjust the pH of the solution to 1 with concentrated sulfuric acid. Weigh 166.83 grams of ferrous sulfate heptahydrate according to the molar ratio of iron to arsenic of 1.5, add it into the solution and keep the solution stirring. Oxygen was bubbled into the solution at a flow rate of 0.5 L / min. The solution was heated to 90 degrees Celsius, kept stirring and reacting with oxygen for 7 hours. After the solution was cooled, it was filtered, the precipitate was washed with deionized water, and dried to obtain a light green powder solid. And the arsenic content in the solution after the reaction was tested by direct reading plasma spectrometer (ICP), and the arsenic deposition rate was calculated to be 99.6%. Soak the obtained solid powder in a sodium hydroxide solution with a pH of 11 for 12 hours, filter, wash, and dry to obtain a khaki powder, which is the target product. Finall...
Embodiment 2
[0028] Prepare 1 liter of 10 g / L arsenic-containing solution according to the above method, and adjust the pH of the solution to 1 with concentrated sulfuric acid. Weigh 74.15 grams of ferrous sulfate heptahydrate according to the molar ratio of iron to arsenic of 2, add it into the solution and keep the solution stirring. Oxygen was bubbled through the solution at a flow rate of 2 liters / minute. The solution was heated to 80 degrees Celsius, kept stirring and reacted with oxygen for 9 hours. After the solution was cooled, it was filtered, the precipitate was washed with deionized water, and dried to obtain a light green powder solid. And the arsenic content in the solution after the reaction was tested by direct reading plasma spectrometer (ICP), and the arsenic deposition rate was calculated to be 99.4%. Soak the obtained solid powder in a sodium hydroxide solution with a pH of 13 for 6 hours, filter, wash, and dry to obtain a light red powder, which is the target product....
Embodiment 3
[0030] Prepare 1 liter of 50 g / L arsenic-containing solution according to the above method, and adjust the pH of the solution to 2 with concentrated sulfuric acid. Weigh 397.62 grams of ferrous chloride tetrahydrate according to the molar ratio of iron to arsenic of 3, add it into the solution and keep the solution stirring. Air was bubbled through the solution at a flow rate of 5 liters / minute. The solution was heated to 70 degrees Celsius, kept stirring and reacting with oxygen for 12 hours. After the solution was cooled, it was filtered, the precipitate was washed with deionized water, and dried to obtain a light green powder solid. And the arsenic content in the solution after the reaction was tested by direct reading plasma spectrometer (ICP), and the arsenic deposition rate was calculated to be 99.5%. Soak the obtained solid powder in a sodium hydroxide solution with a pH of 14 for 2 hours, filter, wash, and dry to obtain a red powder, which is the target product.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com