A kind of synthetic method of dye compound red base kd
A dye compound and synthesis method technology, applied in the field of dye compound synthesis, can solve the problems of long reaction process, long cycle, toxicity, etc., and achieve the effects of improving production efficiency, avoiding use and emission, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Its specific operation is as follows:
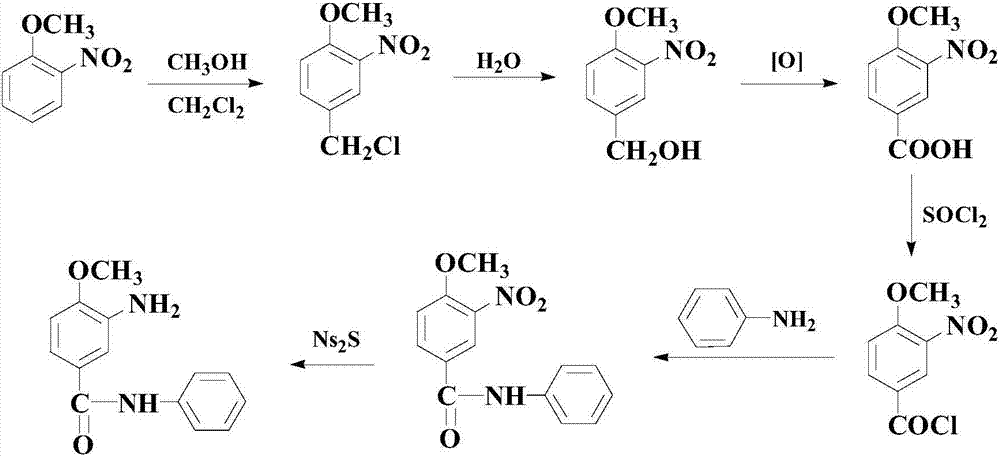
[0048] S1: at room temperature, add 100mmol of the following formula (I) compound 4-methoxy-3-aminobenzyl alcohol, 150mmol of the following formula (II) compound, 10mmol composite catalyst (for 5mmol 1-di A mixture of phenylphosphino-1'-(di-tert-butylphosphino)ferrocene and 5 mmol bis(cyclopentadienyl) zirconium dichloride), 120 mmol oxidant cerium ammonium nitrate and 8 mmol organic ligand L1, and then The temperature was raised to 80°C, and the reaction was stirred at this temperature for 10 hours;
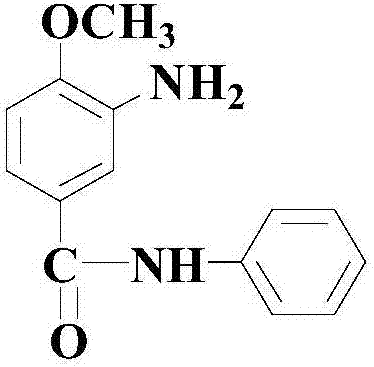
[0049] S2: After the reaction of step S1 is completed, directly add 300 mmol of reducing agent Na 2 S (being the aqueous solution form of mass percent concentration 30%), and continue to react at 80 ℃ for 14 hours, after finishing, through aftertreatment, thereby obtain the following formula (III) compound (being red base KD) for white powder, The yield was 93.8%.
[0050] The specific reaction formula and process are as follows: ...
Embodiment 2
[0055] Its specific operation is as follows:
[0056] S1: at room temperature, add 100mmol of the formula (I) compound 4-methoxy-3-aminobenzyl alcohol, 250mmol of the formula (II) compound, 6mmol composite catalyst (3mmol 1 - a mixture of diphenylphosphino-1'-(di-tert-butylphosphino)ferrocene and 3 mmol bis(cyclopentadienyl) zirconium dichloride), 160 mmol oxidant cerium ammonium nitrate and 4 mmol organic ligand L1 , then warming up to 100°C, and stirring and reacting at this temperature for 6 hours;
[0057] S2: After the reaction in step S1 is completed, directly add 400 mmol of reducing agent NaHS (in the form of an aqueous solution with a mass percentage concentration of 50%), and continue to react at 100° C. for 10 hours, and after the end, undergo post-treatment to obtain a white powder The compound of formula (III) (ie the red base KD) has a yield of 93.5%.
[0058] The reaction formula and flow process as well as various characterization data are the same as in Exam...
Embodiment 3
[0060] Its specific operation is as follows:
[0061] S1: at room temperature, add 100mmol described formula (I) compound 4-methoxy-3-aminobenzyl alcohol, 200mmol described formula (II) compound, 8mmol composite catalyst (being 4mmol 1 - a mixture of diphenylphosphino-1'-(di-tert-butylphosphino)ferrocene and 4 mmol bis(cyclopentadienyl) zirconium dichloride), 140 mmol oxidant ceric ammonium nitrate and 6 mmol organic ligand L1 , then warming up to 90°C, and stirring and reacting at this temperature for 8 hours;
[0062] S2: After the reaction in step S1 is completed, directly add 350 mmol of reducing agent Na 2 S (in the form of an aqueous solution of 40% concentration by mass), and continued to react at 90°C for 12 hours, and after finishing, the compound of formula (III) was obtained as a white powder (i.e. the red base KD) , and the yield was 93.6%.
[0063] The reaction formula and flow process as well as various characterization data are the same as in Example 1, and w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com