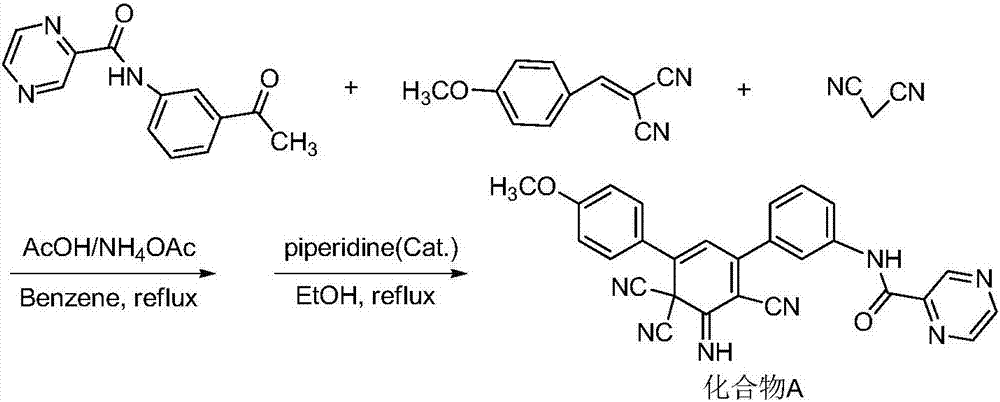
A kind of synthetic method of 3,5-diaryl-2,6,6-tricyano-1-imino-2,4-cyclohexadiene derivative
A synthesis method and a diaryl technology are applied in the field of synthesis of 1-iminocyclohexadiene derivatives, which can solve the problems of low yield and the like, and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Taking the synthesis of 3,5-diphenyl-2,6,6-tricyano-1-imino-2,4-cyclohexadiene with the following structural formula as an example, the specific synthesis method is as follows:
[0031]
[0032] 1. Add 0.4161g (2mmol) 1,3-diphenylpropenone-1, 0.2642g (2mmol) 2-amino-1,1,3-tricyanopropene, 0.4246g to a 50mL dry single-necked flask in sequence (2mmol)K 3 PO 4 , 5mL of methanol, stirred at room temperature for 2 hours, added 20mL of ethyl acetate, the mixture was washed three times with saturated brine, the organic phase was dried overnight with anhydrous sodium sulfate, filtered to remove the desiccant, concentrated under reduced pressure, the crude product was separated by silica gel column chromatography (with Petroleum ether and ethyl acetate with a volume ratio of 4:1 is the eluent), concentrated under reduced pressure, and dried in vacuo to obtain 1,3-diphenyl-3-(2-amino-1,1, 3-Tricyanoallyl)acetone-1, 95% yield.
[0033] 2. Add 0.3404g (1mmol) 1,3-diphenyl-3-(2-...
Embodiment 2
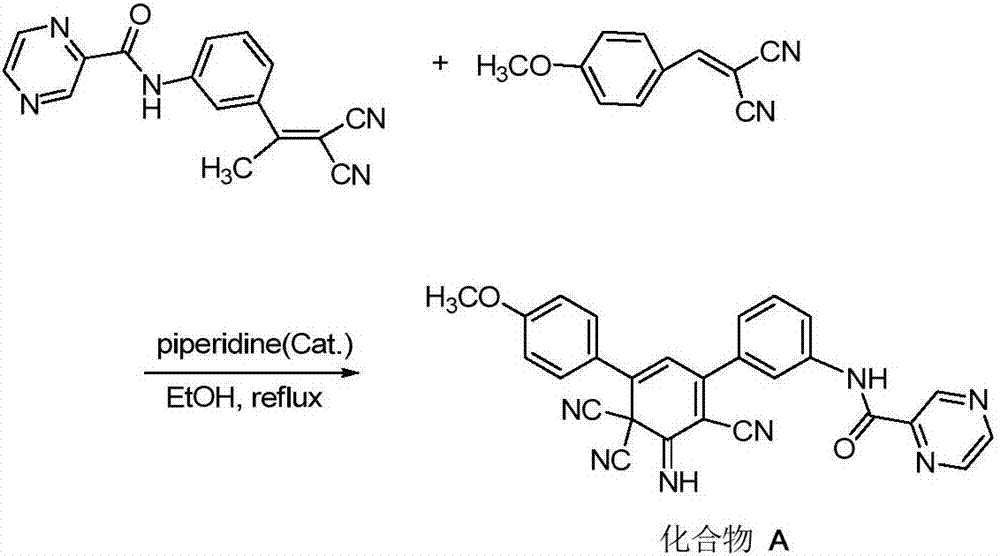
[0038] Taking the synthesis of 3-(4-fluorophenyl)-5-phenyl-2,6,6-tricyano-1-imino-2,4-cyclohexadiene as an example, the specific synthesis method is :
[0039]
[0040] In step 1 of Example 1, the 1,3-diphenylpropenone-1 used was replaced with equimolar 1-phenyl-3-(4-fluorophenyl)-propenone-1, and the reaction time was prolonged to 5 hours, the other steps were the same as in Example 1 to obtain brick red powder 3-(4-fluorophenyl)-5-phenyl-2,6,6-tricyano-1-imino-2,4- Cyclohexadiene, the yield is 78%, the melting point>300°C (ethyl acetate), and the structural characterization data are as follows:
[0041] 1 H NMR (400MHz, DMSO) δ (ppm): 8.13 (dd, J=6.6, 3.1Hz, 2H), 7.67 (dd, J=8.7, 5.4Hz, 2H), 7.49 (d, J=1.7Hz, 1H ), 7.48 (d, J=1.9Hz, 2H), 7.36 (t, J=8.9Hz, 2H), 7.15 (s, 1H).
[0042] 13 C NMR (100MHz, DMSO) δ (ppm): 161.57, 160.94, 156.57, 154.45, 137.19, 130.88, 130.05(2), 128.56(2), 127.01(2), 122.12, 116.88, 115.54(3), 115.33( 2), 109.75, 91.99, 30.06.
[0043] H...
Embodiment 3
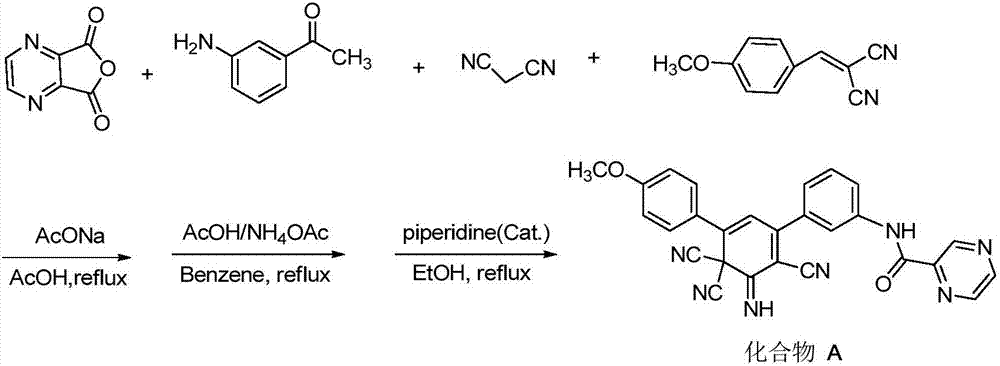
[0045] To synthesize 3-(4-fluorophenyl)-5-(4-methoxyphenyl)-2,6,6-tricyano-1-imino-2,4-cyclohexadiene with the following structural formula For example, the specific synthesis method is:
[0046]
[0047] In step 1 of Example 1, the 1,3-diphenylpropenone-1 used was equimolar with 1-(4-methoxyphenyl)-3-(4-fluorophenyl)-propenone -1 replacement, the reaction time was extended to 5 hours, other steps were the same as in Example 1, and brown powder 3-(4-fluorophenyl)-5-(4-methoxyphenyl)-2,6,6- Tricyano-1-imino-2,4-cyclohexadiene, the yield is 89%, the melting point is >300°C (ethyl acetate), and the structural characterization data are as follows:
[0048] 1 H NMR (400MHz, DMSO) δ (ppm): 8.14 (d, J = 8.9Hz, 2H), 7.65 (dd, J = 8.7, 5.5Hz, 2H), 7.35 (t, J = 8.8Hz, 2H), 7.09 (s, 1H), 7.03 (d, J=8.9Hz, 2H), 3.82 (s, 3H).
[0049] 13 C NMR (100MHz, DMSO) δ (ppm): 163.36, 161.66, 161.16, 160.60, 156.25, 154.02, 134.26, 130.82(2), 129.83, 128.66, 122.49, 117.13(3), 115.42(2), 113...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com