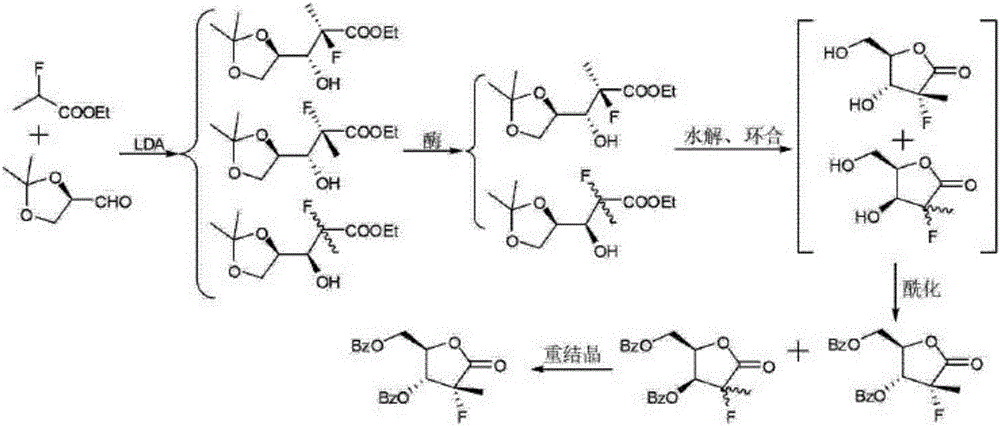
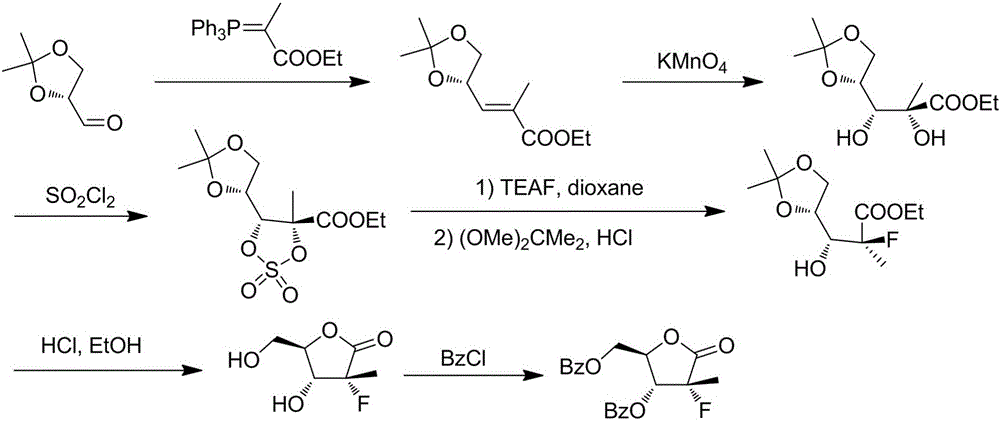
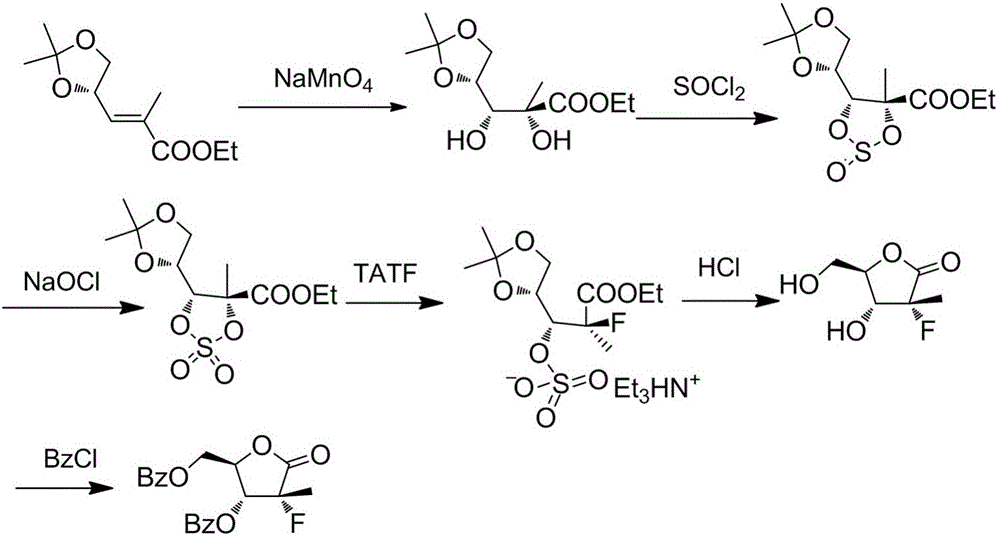
Preparation method for 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribono-gamma-lactone
A technology of dibenzoyl and methyl groups, applied in the field of medicine, can solve the problems of difficulty in purifying starting materials, corrosion of enamel kettle equipment, and incompatibility with large-scale production, and achieves alleviation of environmental pressure in factories, low prices, and three wastes small amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] The present invention will be described below with specific examples. It should be understood that examples are used to illustrate the present invention rather than to limit the present invention, and the scope and core content of the present invention are determined according to the claims.
[0047] A preparation method of 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribose-γ-lactone of the present invention specifically comprises the following steps:
[0048] 1) First add 500L of acetone to the reaction kettle, then add 100kg of D-mannitol and 1kg of stannous chloride in batches, and stir for 1 hour. Add 120kg of 2,2-dimethoxypropane under temperature control at 20°C-25°C, raise the temperature to 35-40°C and react for 5 hours. After the reaction was completed, the acetone was removed by concentration under reduced pressure, and the residue was added with dichloromethane and stirred evenly. About 500 L of saturated aqueous sodium carbonate solution was added to another ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com