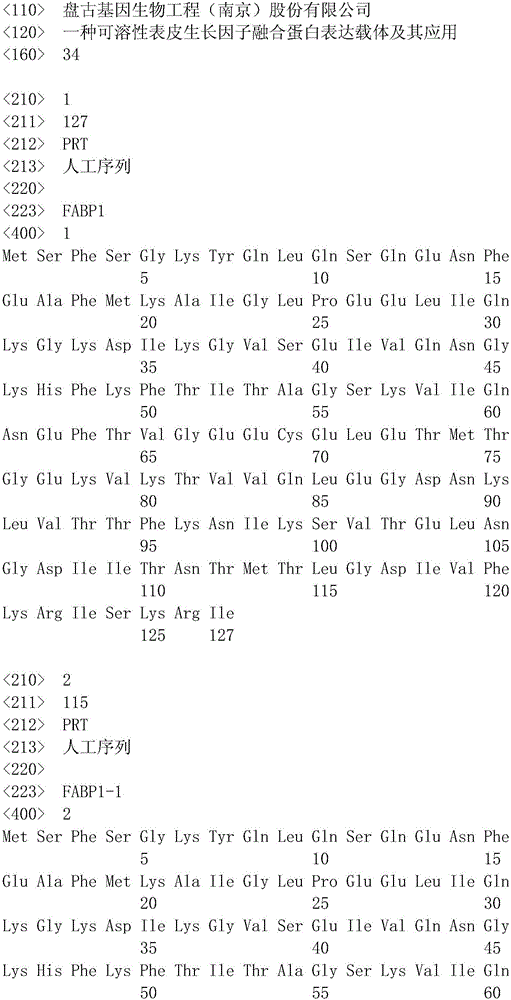Expression vector for soluble epidermal growth factor fusion protein and application thereof
An epidermal growth factor and fusion protein technology, applied in epidermal growth factor, growth factor/inducing factor, animal/human protein, etc., can solve the problem of easy mismatch, incomplete in vitro renaturation, EGF cannot reach pharmaceutical grade activity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
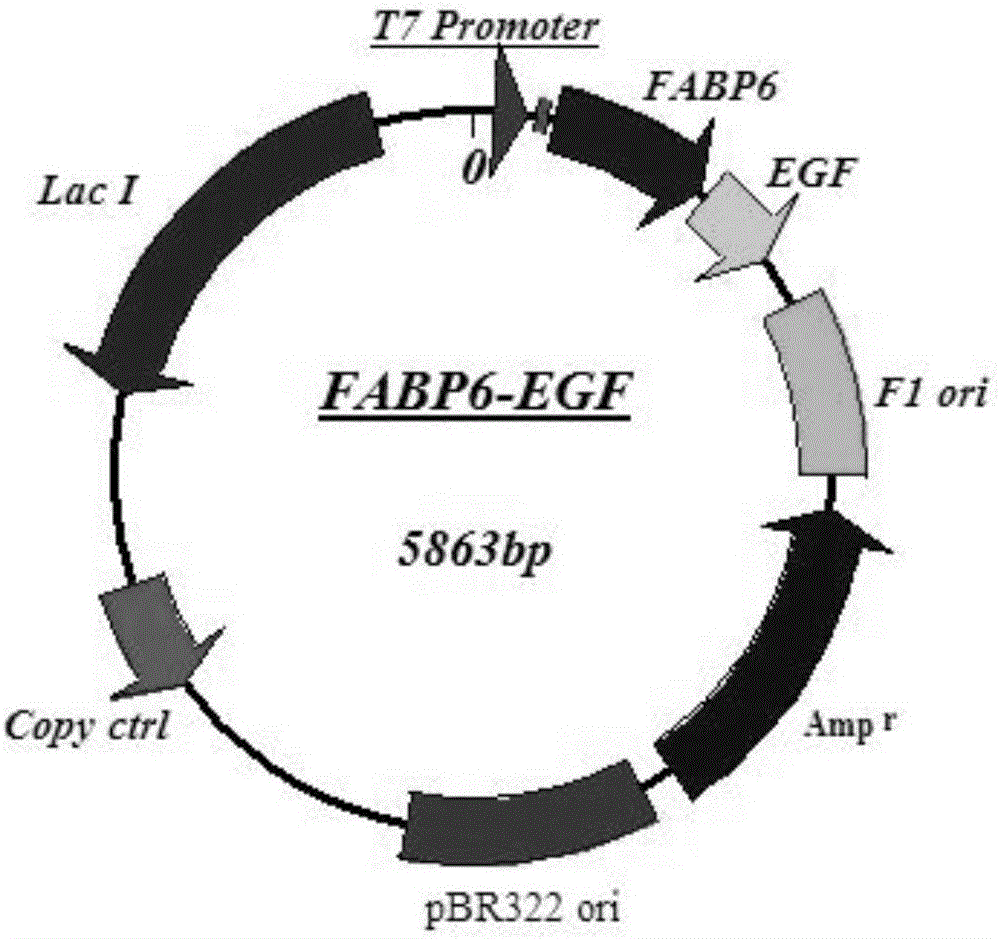
[0053] A method for constructing a soluble epidermal growth factor fusion protein FABP6-EGF expression vector, comprising the following steps:
[0054] Step 1. Artificial synthesis and optimization of DNA encoding human FABP and human EGF. In this embodiment, human FABP6 is taken as an example (SEQ ID NO:8), and human EGF is taken as an example of a mature peptide with 53 amino acids (SEQ ID NO:12 ):
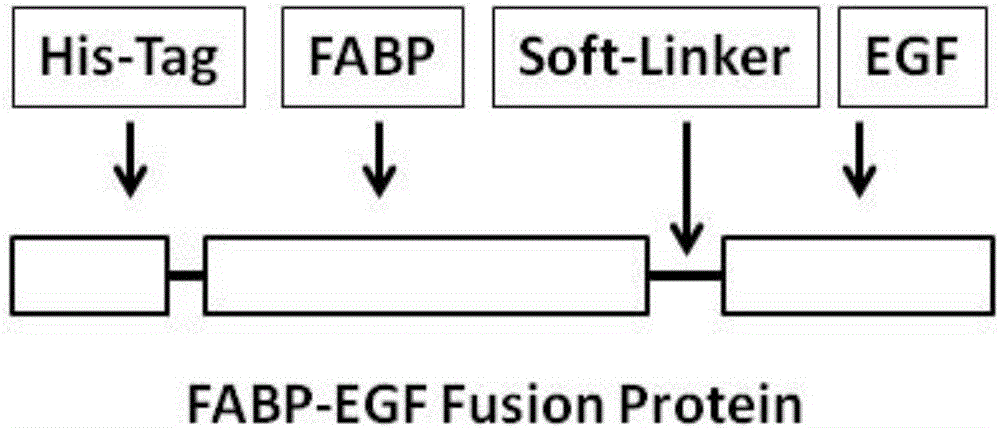
[0055] (1) His-Tag amino acid sequence (SEQ ID NO: 13), FABP6 amino acid sequence (SEQ ID NO: 14) with the initial methionine removed, flexible linker amino acid sequence (SEQ ID NO: 15), EGF amino acid sequence The sequence (SEQ ID NO: 12) is combined into "His-Tag-FABP6-flexible linker-EGF" (SEQ ID NO: 16) in order, but not limited to this sequence, the protein amino acid sequence homology is equal to or greater than 85% limit.
[0056] (2) Input the coding sequence (SEQ ID NO:16) into NCBI software DNAworks (v3.2.3):
[0057] ① Obtain the reverse-translated and codon-optim...
Embodiment 2
[0084] The expression test of embodiment 2 vector:
[0085] (1) The obtained pFABP6-EGF vector DNA plasmid was transformed into competent cells of Escherichia coli expression strain BL21 (DE3) to obtain kanamycin (Kan) resistant colonies.
[0086] (2) Pick a few single colonies, culture them in LB medium, induce with IPTG for 4-12 hours, collect 1 mL of the bacterial liquid, collect the bacterial cells by centrifugation, wash once with 1×PBS, resuspend in 0.5 mL 1×PBS, and sonicate the bacteria body, centrifuged to remove the precipitate.
[0087] (3) Mix an appropriate amount of 20 μL of supernatant with SDS-PAGE loading buffer, heat and denature at 95°C for 10 minutes, collect by centrifugation to the bottom of the tube, and place on ice.
[0088] (4) 10 μL of the sample was taken, electrophoresed with 12% polyacrylamide, stained with Coomassie brilliant blue, and decolorized, and the protein band and the expression of FABP6-EGF protein were observed.
[0089] (5) Recombin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com