Method for dynamically evaluating sulfur removal efficiency of triazine sulfur removal agent
A technique for evaluating methods and efficiency, which is applied in the direction of chemical analysis by titration, and can solve problems such as lack of evaluation of desulfurizers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1, the determination of sulfur removal efficiency of sulfur removal agent, all percentage concentrations are mass percentages, and the water used is distilled water.
[0021] A method for dynamically evaluating sulfur removal efficiency of a sulfur removal agent, comprising the following steps:
[0022] 1) Prepare 400g / L sodium sulfide solution, 100g / L zinc acetate solution, 100g / L NaOH solution, 6mol / L hydrochloric acid solution and 10% triazine desulfurizer;
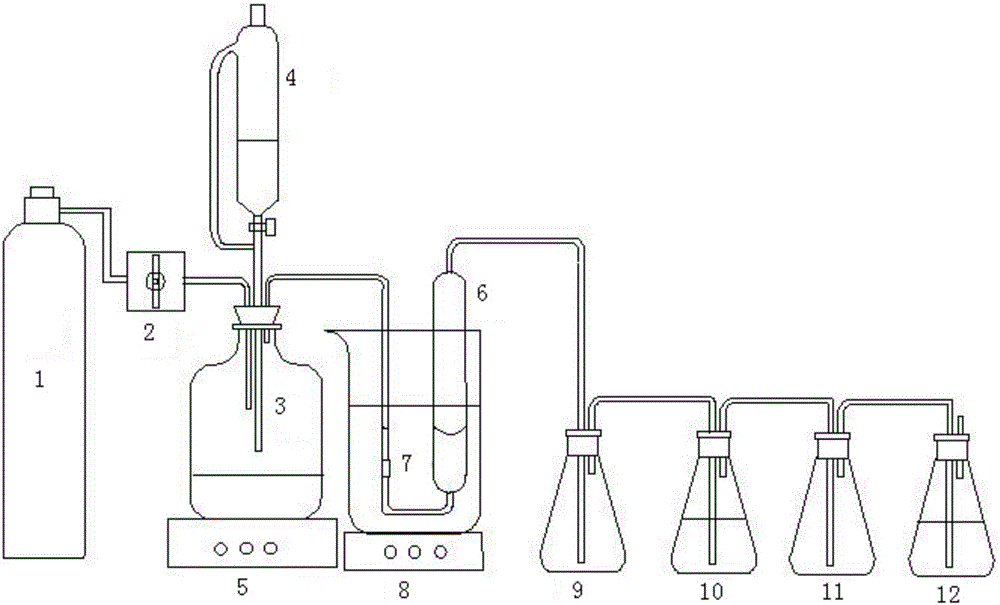
[0023] 2) Connect the instrument, refer to figure 1 , the outlet pipe of the nitrogen cylinder 1 is communicated with the inlet pipe of the sodium sulfide reaction bottle 3 through the rotameter 2, the sodium sulfide reaction bottle 3 is provided with a constant pressure separatory funnel 4, and the bottom of the sodium sulfide reaction bottle 3 is provided with a magnetic stirrer 5, the gas outlet pipe of the sodium sulfide reaction bottle 3 communicates with the bottom air intake pipe of the desulfuri...
Embodiment 2
[0029] Example 2, the determination of sulfur removal efficiency of sulfur removal agent, all percentage concentrations are mass percentages, and the water used is distilled water.
[0030] A method for dynamically evaluating sulfur removal efficiency of a sulfur removal agent, comprising the following steps:
[0031] 1) Prepare 400g / L sodium sulfide solution, 100g / L zinc acetate solution, 100g / L NaOH solution, 6mol / L hydrochloric acid solution and 20% triazine desulfurizer;
[0032] 2) Connect the instrument, refer to figure 1 , the outlet pipe of the nitrogen cylinder 1 is communicated with the inlet pipe of the sodium sulfide reaction bottle 3 through the rotameter 2, the sodium sulfide reaction bottle 3 is provided with a constant pressure separatory funnel 4, and the bottom of the sodium sulfide reaction bottle 3 is provided with a magnetic stirrer 5, the gas outlet pipe of the sodium sulfide reaction bottle 3 communicates with the bottom air intake pipe of the desulfuri...
Embodiment 3
[0038] Example 3, the determination of sulfur removal efficiency of sulfur removal agent, all percentage concentrations are mass percentages, and the water used is distilled water.
[0039] A method for dynamically evaluating sulfur removal efficiency of a sulfur removal agent, comprising the following steps:
[0040] 1) Prepare 400g / L sodium sulfide solution, 100g / L zinc acetate solution, 100g / L NaOH solution, 6mol / L hydrochloric acid solution and 50% triazine desulfurizer;
[0041] 2) Connect the instrument, refer to figure 1, the outlet pipe of the nitrogen cylinder 1 is communicated with the inlet pipe of the sodium sulfide reaction bottle 3 through the rotameter 2, the sodium sulfide reaction bottle 3 is provided with a constant pressure separatory funnel 4, and the bottom of the sodium sulfide reaction bottle 3 is provided with a magnetic stirrer 5, the gas outlet pipe of the sodium sulfide reaction bottle 3 communicates with the bottom air intake pipe of the desulfuriz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com