Highly glycosylated exendin-4 and its analogue fusion protein, its preparation method and use
A fusion protein and analogue technology, applied in biochemical equipment and methods, chemical equipment and methods, animal/human proteins, etc., can solve the problems of short half-life and strong immunogenicity of Exendin-4
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Embodiment 1, construct the expression plasmid encoding fusion protein
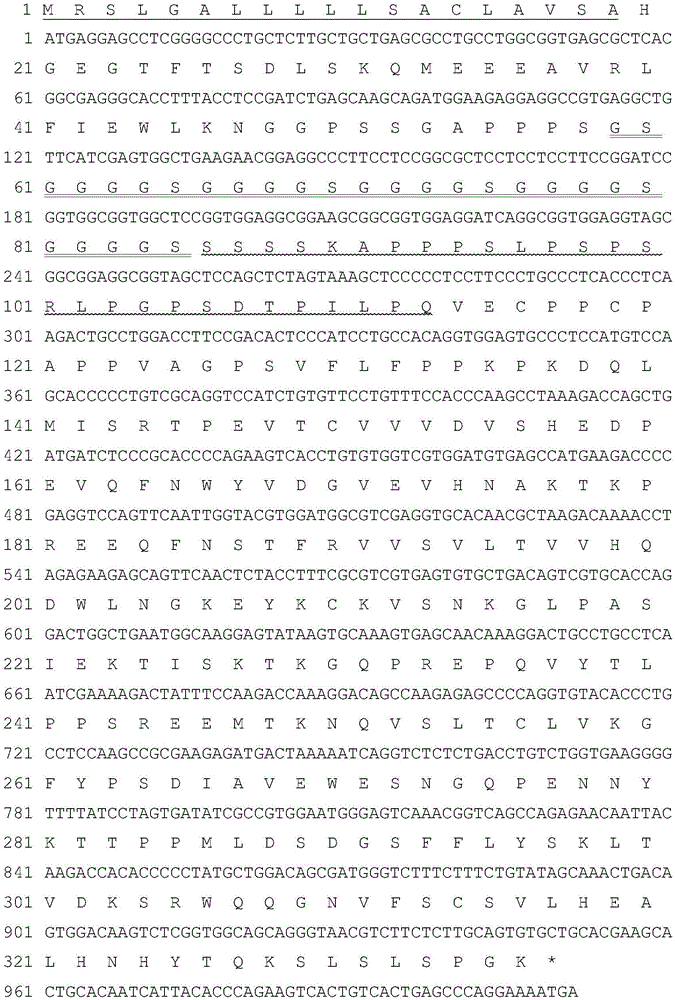
[0120] The gene sequences encoding α1 microglobulin (α1 microglobulin) leader peptide and mature Exendin-4 and its analogues, flexible peptide linker, CTP rigid unit and human IgG Fc variants are all artificially optimized CHO cell preferred codons. Long sequences were obtained by chemical synthesis. In order to facilitate the insertion of the target fragment into the specific site of the expression vector, there is a restriction enzyme site at the 5' and 3' ends of the synthesized fragment, respectively SpeI and EcoRI. The verified Exendin-4 and its analog fusion protein genes were digested with SpeI and EcoRI, and then inserted into the corresponding restriction sites of the plasmid PXY1A1 transformed by PCDNA3.1 to obtain the fusion gene expression plasmid. The plasmid contains the early promoter of cytomegalovirus, which is an enhancer required for high-level expression of foreign genes in mam...
Embodiment 2
[0124] Embodiment 2, the expression of fusion protein in transfection cell line
[0125] The recombinant expression vector plasmid is transfected into a mammalian host cell line to express the fusion protein of Exendin-4 and its analogues. In order to stably express at a high level, the preferred host cell line is DHFR enzyme-deficient CHO-cells (see US Pat. No. 4,818,679). In this example, the host cell line is the CHO-derived cell line DXB11. A preferred method of transfection is electroporation, although other methods including calcium phosphate co-sedimentation, lipofection, and protoplast fusion can also be used. In electroporation, use a Gene Pulser electroporator (Bio-Rad Laboratories, Hercules, CA) set to a 300V electric field and a capacitance of 1050 μFd, in a 5×10 cell in a cuvette. 7 Add 50 μg of high-purity expression plasmid to each cell. Two days after transfection, the medium was changed to growth medium containing 0.6 mg / mL G418. Transfectants were screened...
Embodiment 3
[0127] Embodiment 3, purification and qualitative of fusion protein
[0128] The conditioned medium containing the fusion protein was titrated to pH 7-8 with 1N NaOH and filtered through a 0.45 micron nitrocellulose filter. The filtrate was loaded onto a Protein A column equilibrated in phosphate buffered saline (PBS). After the fusion protein is bound to the Protein A column, discard the effluent fraction. The column was washed with PBS until the OD value at 280nm was below 0.01. The bound fusion protein was then eluted with 0.1M citrate buffer, pH 3.75. Eluate with 0.4 volumes of 1MK 2 HPO 4 For neutralization, fractions containing purified protein were pooled and dialyzed against PBS. Then filter through a 0.22 μm nitrocellulose filter and store at 4 °C. Under non-reducing and reducing conditions, the protein products were identified and analyzed by SDS-PAGE. The fusion protein was quantified by BCA protein assay using BSA as a standard.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com