Application of isoquinoline alkaloid to prevention or treatment of diabetes
A technology for isoquinoline alkaloids and diabetes, which is applied in the field of natural medicines and can solve problems such as no preventive or therapeutic effects of phellodendri
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
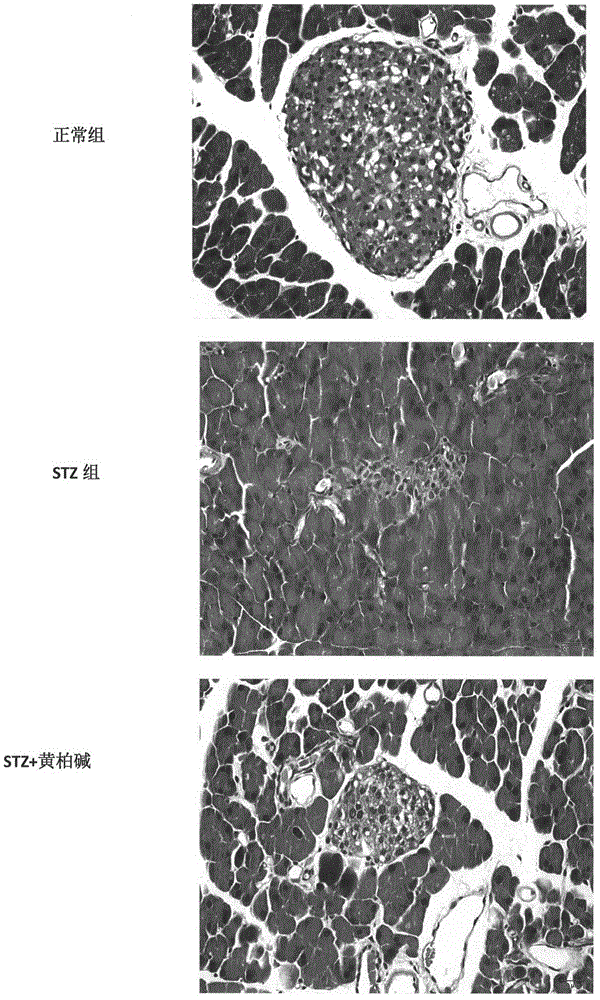
[0070] In order to study the effect of cortex base on the blood sugar of type I diabetes, this experiment adopts the classic type I diabetes model, and uses C57BL mice to be randomly divided into 3 groups, normal control group (10 rats), STZ group (20 rats). In the STZ group, STZ (50mg / Kg) was injected intraperitoneally continuously for 5 days, and the fasting blood glucose was measured two weeks later. If the blood glucose was greater than or equal to 13.8mmol / L, the modeling was successful. Then STZ group is randomly divided into STZ group and Phellodendron treatment group, every group of 10, Phellodendron treatment group adopts oral gavage (15mg / kg) after 10 weeks, measures fasting blood glucose result after fasting 4h as follows: figure 1 showed a statistically significant difference.
[0071] General condition of the animal:
[0072] Histopathological examination of pancreatic islets: paraffin section, HE staining, observation of pathological changes
[0073] Table 1. T...
Embodiment 2
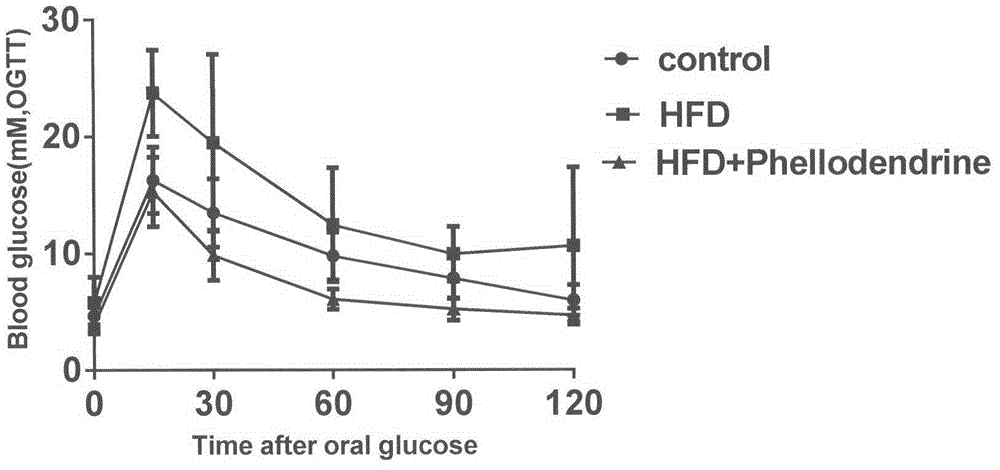
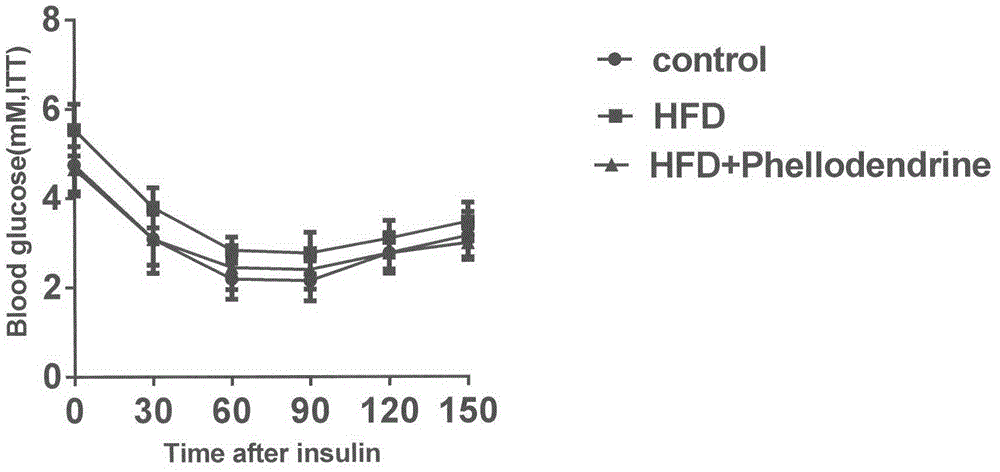
[0082] In order to study the influence of Phellodendronine on type II diabetes, this experiment adopts the classic Type II diabetes model, and uses C57BL mice to be randomly divided into 3 groups, normal control group (10 mice), model group (10 mice), and Phellodendronine treatment group. (10), wherein the model group and the cortex base treatment group adopt high-fat diet (basic feed adds 20% lard) to make models for 8 weeks, and the cortex base treatment group adopts oral gavage (50mg / kg) mode while high-fat diet After administration, fasting overnight assay, fasting blood glucose, glucose tolerance and insulin tolerance were measured after administration. The experimental data were subjected to analysis of variance, and the results were expressed as x±s. The results showed that Phellodendronine treatment effect was obvious, with significant statistical difference.
[0083] Experimental results:
[0084] Table 3. Effects of Phellodendronine on Fasting Blood Glucose in Type...
Embodiment 3
[0119] Therapeutic effect of cortex base on hyperlipidemia and non-alcoholic fatty liver,
[0120] 1. Experimental animals and methods:
[0121] C57BL mice, SPF grade, male, body weight (20 ± 2) g, were randomly divided into two groups, the first group of 12 was the normal group, and was given normal feed, and the remaining mice (44) were divided into the second group, Give high-fat feed (basic feed with 20% lard, 1.25% cholesterol, 0.5% sodium cholate) to eat and drink freely. After 8 weeks of continuous feeding, 4 mice from the normal control group and the fatty liver model group were sacrificed respectively, and the serum and liver biochemical indicators of the two groups of mice were compared, as well as the liver tissue pathological sections, to determine whether the non-alcoholic fatty liver model mice were Created successfully.
[0122] 32 successful modeling mice were randomly divided into 4 groups, which were respectively normal control group (8 mice), model group (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com