Method for preparing 2-chloro-4,6-dimethoxy-1,3,5-triazine
A technology of dimethoxyl and methoxylization, which is applied in the direction of organic chemistry, can solve the problems of large loss of organic solvents and complicated process, and achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
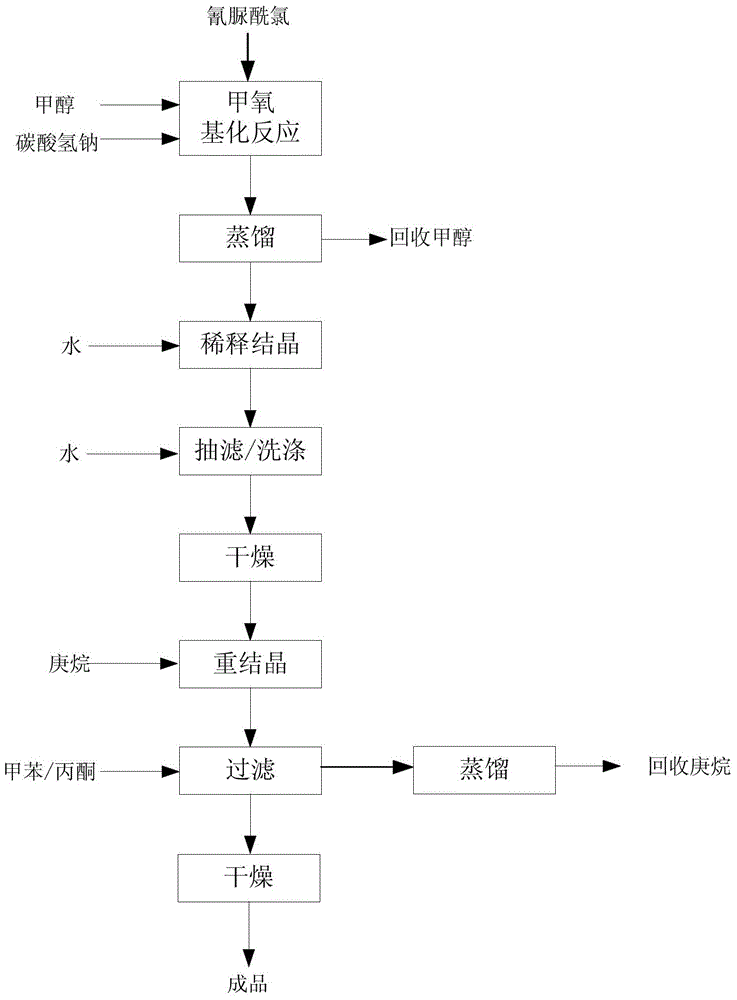
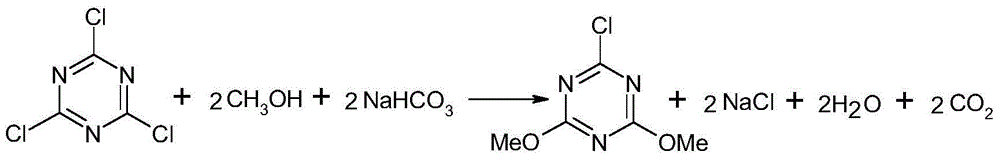
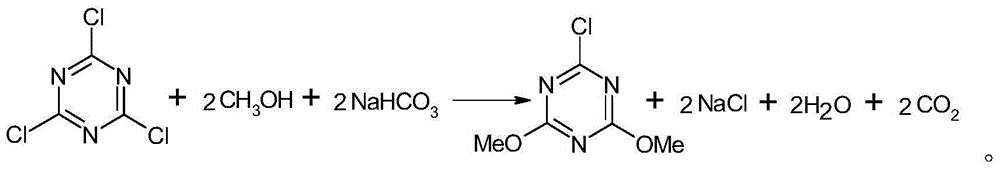
[0021] A method for preparing 2-chloro-4,6-dimethoxy-1,3,5-triazine, comprising the following steps:
[0022] 1) Methoxylation reaction: 900 kg of methanol and 240 kg of sodium bicarbonate were put into the reactor, and 260 kg of cyanuric chloride was slowly added in batches under stirring, and then the temperature was raised to reflux of methanol in stages, and the reaction was carried out for 5 hours;
[0023] 2) Distillation: After the methoxylation reaction, most of the methanol is distilled to recover 750kg; the methanol distillation recovery is carried out in the reactor, and the distillation is condensed by water cooling and freezing brine;
[0024] 3) Dilute crystallization: 516kg of distillation residue is put into the water analysis kettle, and 1010kg of crystallization is diluted with water;
[0025] 4) Suction filtration / washing, drying: adding water to the crystalline solid phase, washing with suction, and drying to obtain 240 kg of crude product;
[0026] 5) Rec...
Embodiment 2
[0029] A method for preparing 2-chloro-4,6-dimethoxy-1,3,5-triazine, comprising the following steps:
[0030] 1) Methoxylation reaction: 900 kg of methanol and 180 kg of sodium bicarbonate were put into the reaction kettle, and 225 kg of cyanuric chloride was slowly added in batches under stirring, and then the temperature was raised to methanol reflux in stages, and the reaction was carried out for 4 hours;
[0031] 2) Distillation: After the methoxylation reaction, most of the methanol is distilled to recover 770kg; the methanol distillation recovery is carried out in the reactor, and the distillation is condensed by water cooling and freezing brine;
[0032] 3) Dilute crystallization: Distill 480kg of residue into the water analysis kettle and add water to dilute crystallization 1010kg;
[0033] 4) Suction filtration / washing, drying: add water to the crystalline solid phase, wash with suction, and dry to obtain 215 kg of crude product;
[0034] 5) Recrystallization, filtra...
Embodiment 3
[0037] A method for preparing 2-chloro-4,6-dimethoxy-1,3,5-triazine, comprising the following steps:
[0038] 1) Methoxylation reaction: 900 kg of methanol and 270 kg of sodium bicarbonate were put into the reactor, and 315 kg of cyanuric chloride was slowly added in batches under stirring, and then the temperature was raised to reflux of methanol in stages, and the reaction was carried out for 6 hours;
[0039] 2) Distillation: After the methoxylation reaction, most of the methanol is distilled to recover 730kg; the methanol distillation recovery is carried out in the reactor, and the distillation is condensed by water cooling and freezing brine;
[0040] 3) Dilute crystallization: 545kg of distillation residue is put into the water analysis kettle and dilutes 1150kg of crystallization with water;
[0041] 4) Suction filtration / washing, drying: add water to the crystalline solid phase, wash with suction, and dry to obtain 275 kg of crude product;
[0042] 5) Recrystallizatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com