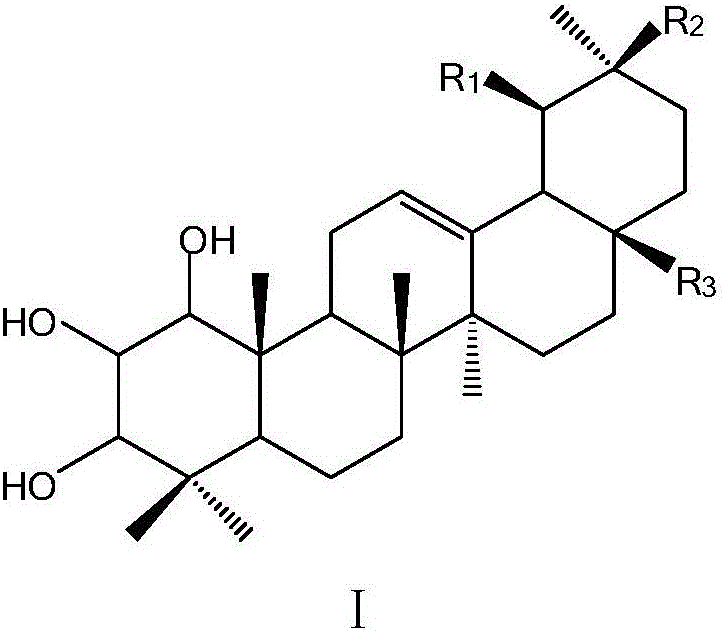
Application of A-cyclo trihydroxy substituted pentacyclic triterpene compounds in pharmaceuticals
A technology of pentacyclic triterpenes and trihydroxyl groups is applied in the application field of preparing antibacterial or anti-tobacco mosaic virus medicines, and can solve the problems that the research on anti-phytopathogenic bacteria and anti-tobacco mosaic virus has not been reported before.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
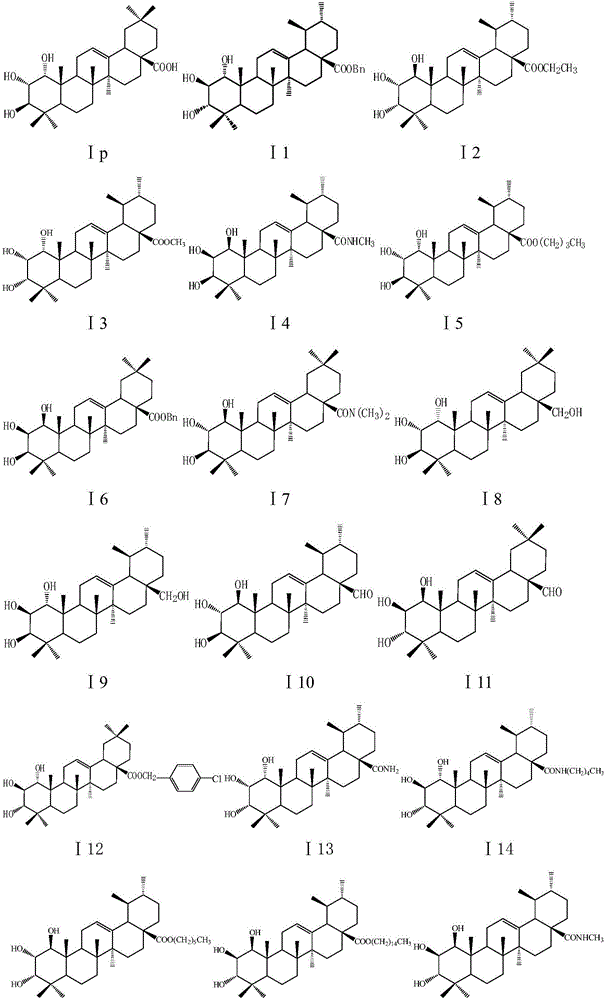
[0163] Embodiment 1 of the present invention: take any one of compound I17, U2, U4, A2, A3, add appropriate amount of starch and microcrystalline cellulose according to the pharmaceutical method, and mix well, mix the hydroxymethyl cellulose solution with the above-mentioned Mix the powder, pass through an 80-mesh sieve to obtain wet granules, dry at 50-60°C, sieve carboxymethyl starch salt, magnesium stearate and talc in advance, and then add them to the above-mentioned granules and press into tablets to obtain tablets . The preparation is taken orally, twice a day, and the dosage for each dose is 5-30 mg calculated as the raw material. For the treatment of infectious diseases caused by Staphylococcus aureus such as purulent infection, pneumonia, pseudomembranous colitis, pericarditis, etc.
Embodiment 2
[0164] Embodiment 2: Take any one of compounds I3, I15, I18, I23, U1, U3, U5, A4, add appropriate amount of sodium citrate, polyethylene glycol, distilled water according to the pharmaceutical method, mix well and fully dissolve, adjust The pH value of the solution is 7.5-8.5 and then filtered, and the concentration of the raw material is 1 mg / ml, which is divided into 2 ml per ampoule and sterilized to obtain the injection. The preparation is used for injection, twice a day, and the amount of each injection is 5-30 mg based on the raw material drug. For the treatment of infectious diseases caused by Staphylococcus aureus.
Embodiment 3
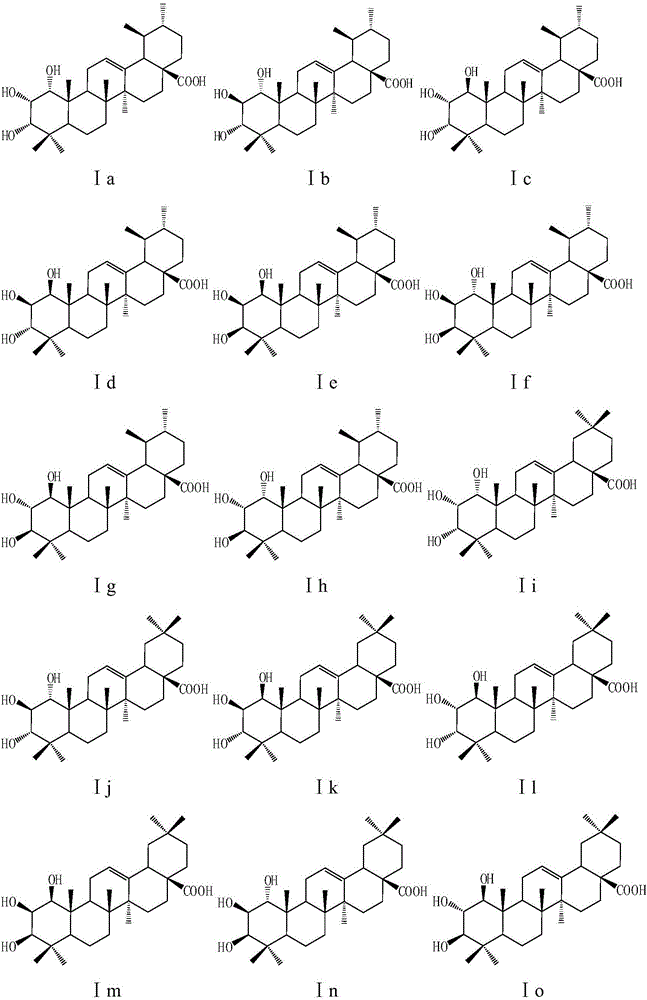
[0165] Example 3: Take any one of compounds Ia, Ic, Ij, Io, Ip, I13, A1, A5, and A7, pulverize it through an 80-mesh sieve, add an appropriate amount of vegetable oil containing 10% beeswax according to the pharmaceutical method, and mix well ; Prepare capsule material with gelatin:glycerol:distilled water:preservative=1:0.4:0.8:0.003, add the mixture of the aforementioned compound and vegetable oil, and press to obtain a soft capsule. The preparation is taken orally, twice a day, and the dosage for each dose is 5-30 mg calculated as the raw material. For the treatment of infectious diseases caused by Staphylococcus aureus.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com