Method for preparing recombinant N-terminal brain natriuretic peptide precursor based on elastin-like label
A technology similar to elastin and brain natriuretic peptide, applied in the field of preparation of recombinant N-terminal brain natriuretic peptide precursor, can solve the problems of high cost and time-consuming, and achieve the effect of high practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
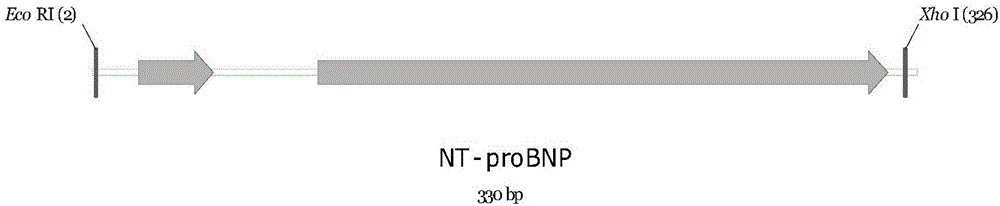
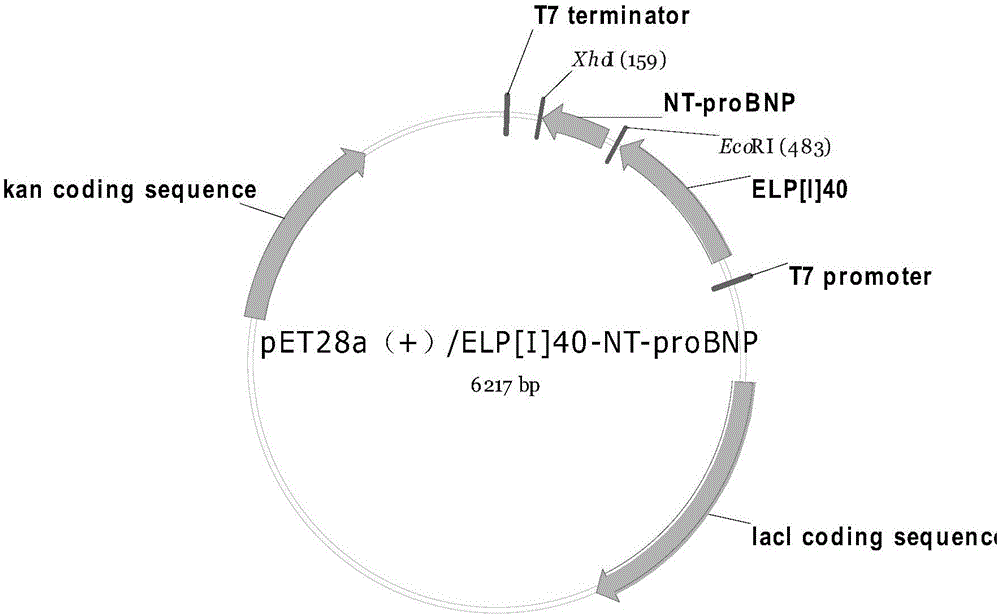
[0033] 1. Construction of NT-proBNP fusion expression vector pET28a(+) / ELP[I] 40 / NT-proBNP. Design and optimize the NT-proBNP gene based on the human BNP gene sequence (EMBL accession number AB037521.1), and entrust Nanjing GenScript Biotechnology Co., Ltd. to synthesize the NT-proBNP gene according to Seq ID.NO.1. The gene structure is as follows figure 1 shown. Cloning NT-proBNP gene into pET28a(+) / ELP[I] by using EcoRI and XhoⅠ restriction sites at both ends of the gene 40On the carrier, form NT-proBNP fusion expression vector pET28a(+) / ELP[I] 40 / NT-proBNP, its nucleotide sequence is Seq ID.NO.2, and the fusion expression vector structure is as follows figure 2 shown.
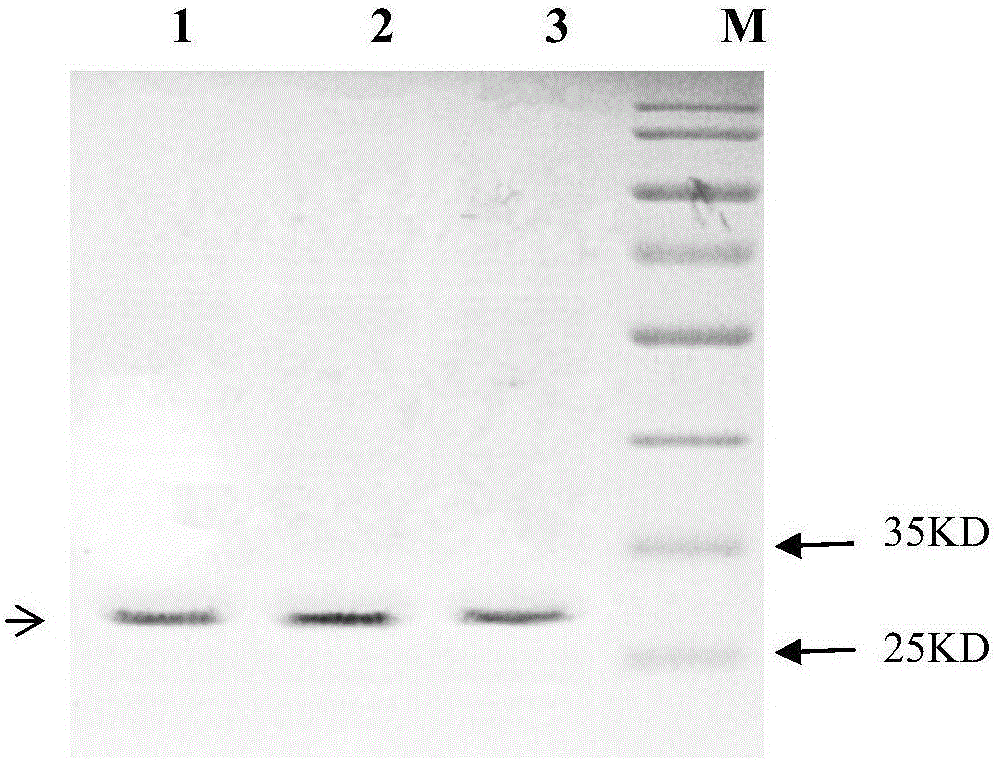
[0034] 2. Transform the BL21(DE3) Escherichia coli strain (purchased from Treasure Bioengineering (Dalian) Co., Ltd.) by electroporation with the constructed expression vector, and inoculate the identified transformant into 10 ml containing kanamycin sulfate (Kan) 50 micrograms per milliliter of LB m...
Embodiment 2
[0040] 1. Construction of NT-proBNP fusion expression vector pET28a(+) / ELP[I] 40 / NT-proBNP. Design and optimize the NT-proBNP gene based on the human BNP gene sequence (EMBL accession number AB037521.1), and entrust Nanjing GenScript Biotechnology Co., Ltd. to synthesize the NT-proBNP gene according to Seq ID.NO.1. The gene structure is as follows figure 1 shown. Cloning NT-proBNP gene into pET28a(+) / ELP[I] by using EcoRI and XhoⅠ restriction sites at both ends of the gene 40 On the carrier, form NT-proBNP fusion expression vector pET28a(+) / ELP[I] 40 / NT-proBNP, its nucleotide sequence is Seq ID.NO.2, and the fusion expression vector structure is as follows figure 2 shown.
[0041] 2. Transform the BL21(DE3) Escherichia coli strain (purchased from Treasure Bioengineering (Dalian) Co., Ltd.) by electroporation with the constructed expression vector, and inoculate the identified transformant into 10 ml containing kanamycin sulfate (Kan) 50 micrograms per milliliter of LB ...
Embodiment 3
[0047] 1. Construction of NT-proBNP fusion expression vector pET28a(+) / ELP[I] 40 / NT-proBNP. Design and optimize the NT-proBNP gene based on the human BNP gene sequence (EMBL accession number AB037521.1), and entrust Nanjing GenScript Biotechnology Co., Ltd. to synthesize the NT-proBNP gene according to Seq ID.NO.1. The gene structure is as follows figure 1 shown. Cloning NT-proBNP gene into pET28a(+) / ELP[I] by using EcoRI and XhoⅠ restriction sites at both ends of the gene 40 On the carrier, form NT-proBNP fusion expression vector pET28a(+) / ELP[I] 40 / NT-proBNP, its nucleotide sequence is Seq ID.NO.2, and the fusion expression vector structure is as follows figure 2 shown.
[0048] 2. Transform the BL21(DE3) Escherichia coli strain (purchased from Treasure Bioengineering (Dalian) Co., Ltd.) by electroporation with the constructed expression vector, and inoculate the identified transformant into 10 ml containing kanamycin sulfate (Kan) 50 micrograms per milliliter of LB ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com