Recombinant protein and application thereof
A recombinant protein and protein technology, applied in the field of recombinant protein and its application, can solve the problems of inability to generate and prepare anti-myocardial infarction and vascular leakage drugs, so as to improve cardiac function, inhibit vascular leakage, and inhibit myocardial cell apoptosis. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0035] Preparation of embodiment 1AGGF1 recombinant protein
[0036] AGGF1 protein expression plasmid pet28-AGGF1 was expressed in Escherichia coli BL21, wherein the sequence of AGGF1 was shown in SEQ NO.1. The expression strain was cultured overnight in 20 ml kanamycin-resistant LB medium. On the next day, add the 20 ml medium to 1 L kanamycin-resistant LB medium, and culture on a shaker at 37° C. for 2.5 hours. Then 1 mM IPTG was added to induce protein expression. IPTG is a highly stable lactose analog that inhibits the lac repressor and induces the synthesis of β-galactosidase. This enzyme promotes the utilization of lactose. IPTG can be used to induce the expression of target genes regulated by the lac operator. After induction by adding IPTG, E. coli continued to grow in the medium for 5.5 hours. Then centrifuge the culture medium, 4000rpm, 4°C, 10mins. If the protein is not to be isolated immediately, store the E. coli pellet at -20°C. The recombinant protein was...
Embodiment 2A
[0037] Example 2 Preparation of AGGF1 Homologous Recombination Protein
[0038] 100%, 99%, 98%, 97%, 96%, 95%, 94%, 93%, 92%, 91%, 90%, 89%, 88%, 87%, Proteins with 86%, 85%, 84%, 83%, 82%, 81% and 81% homology were prepared. The pet28 protein expression plasmid for obtaining AGGF1 homologous protein was constructed, and prepared according to the full-length AGGF1 protein recombination method, and the preparation method was the same as that in Example 1.
Embodiment 1
[0040] The angiogenesis-promoting effect of the recombinant protein synthesized in Example 1 and its application in the preparation of anti-myocardial infarction drugs
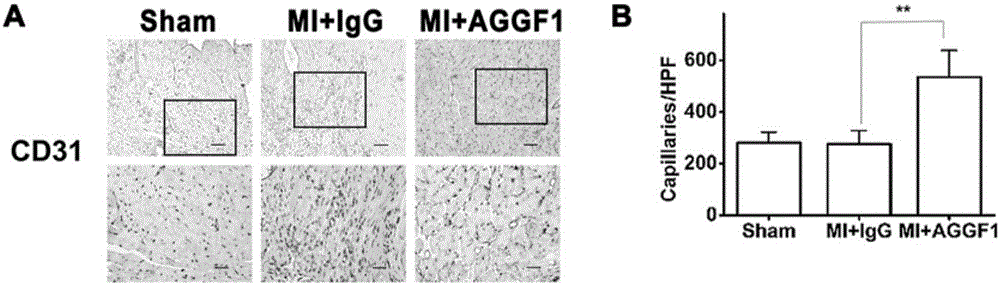
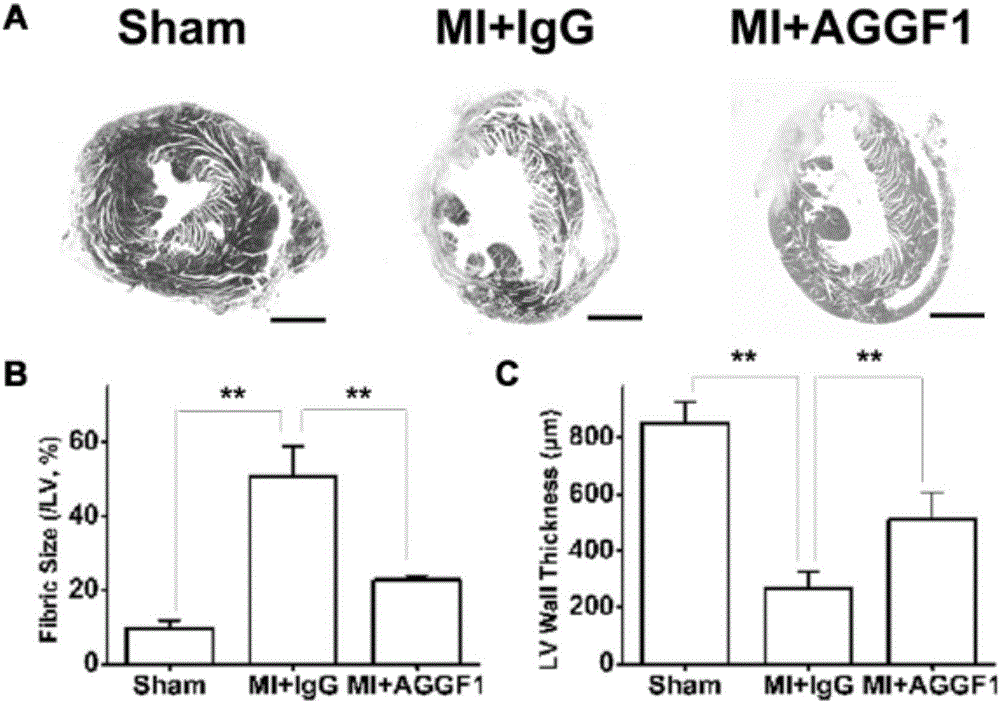
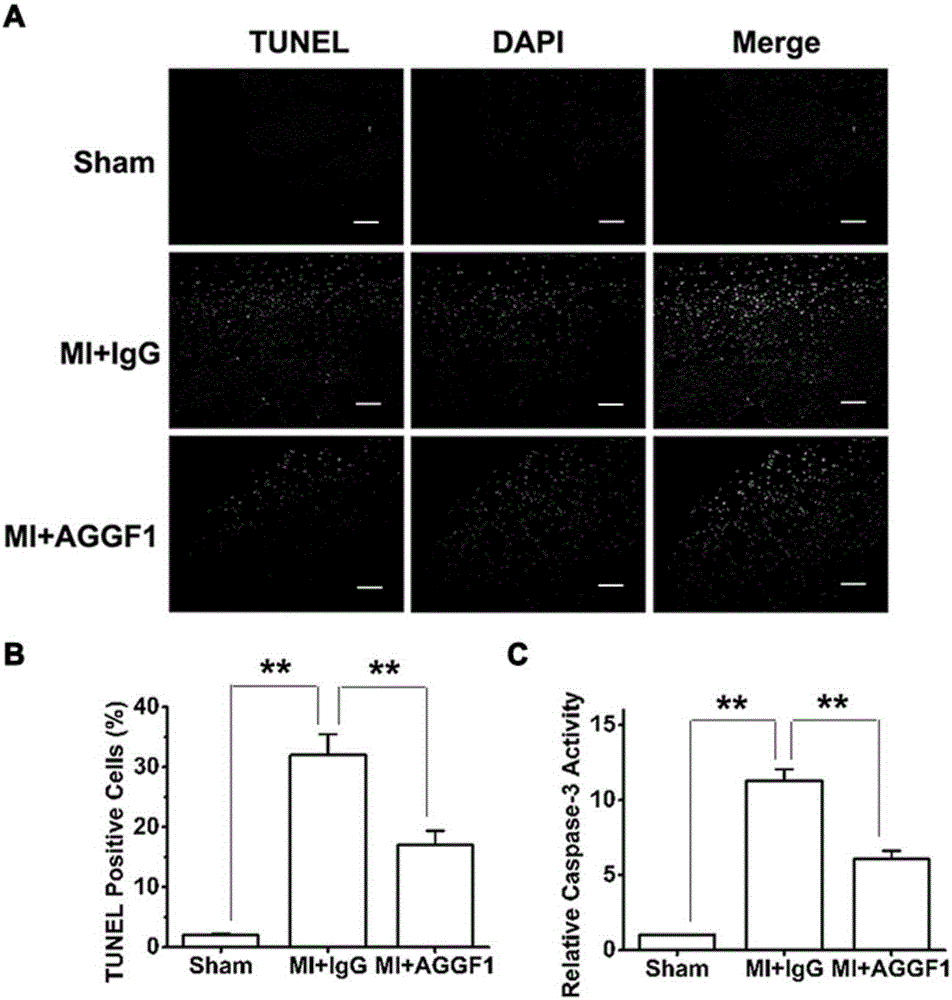
[0041] The C57BL / 6 mice were divided into 3 groups: sham operation group (Sham), placebo group (MI+IgG) for myocardial infarction surgery, recombinant AGGF1 protein group (MI+AGGF1) for myocardial infarction surgery, 12 mice per group Only. Before surgery and after myocardial infarction surgery, the cardiac function of mice was evaluated with a small animal ultrasound imaging system. One day after the operation, the mice were examined by ultrasound to confirm that the myocardial infarction operation was successful. One week after the successful operation, mice were injected with recombinant AGGF1 protein or the same dose of IgG as a control by tail vein, twice a week for 2 weeks. The protein dosage is: 0.25mg / kg.
[0042] Compared the number of new blood vessels (CD31 staining) in the hearts of mice in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com