Low-dose non-steroidal anti-inflammatory drug composition and preparation process thereof
A non-steroidal anti-inflammatory, low-dose technology, used in drug combinations, anti-inflammatory agents, antipyretics, etc., can solve problems such as unsafe and unfavorable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
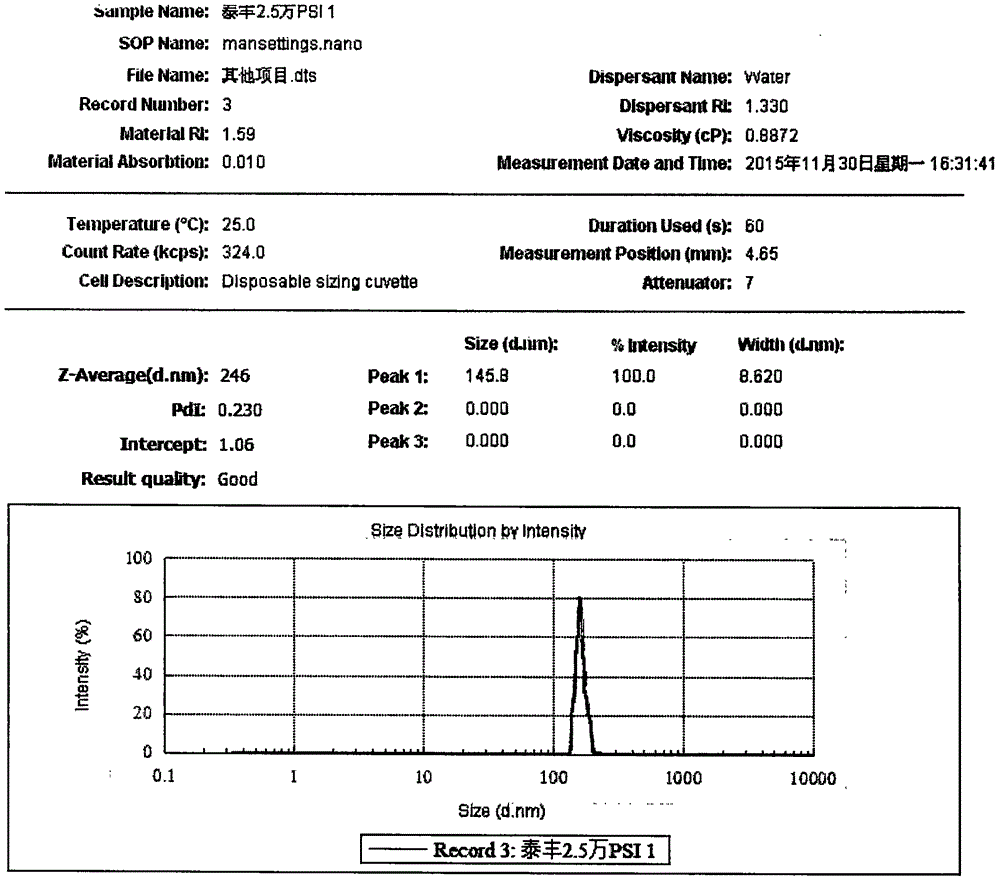
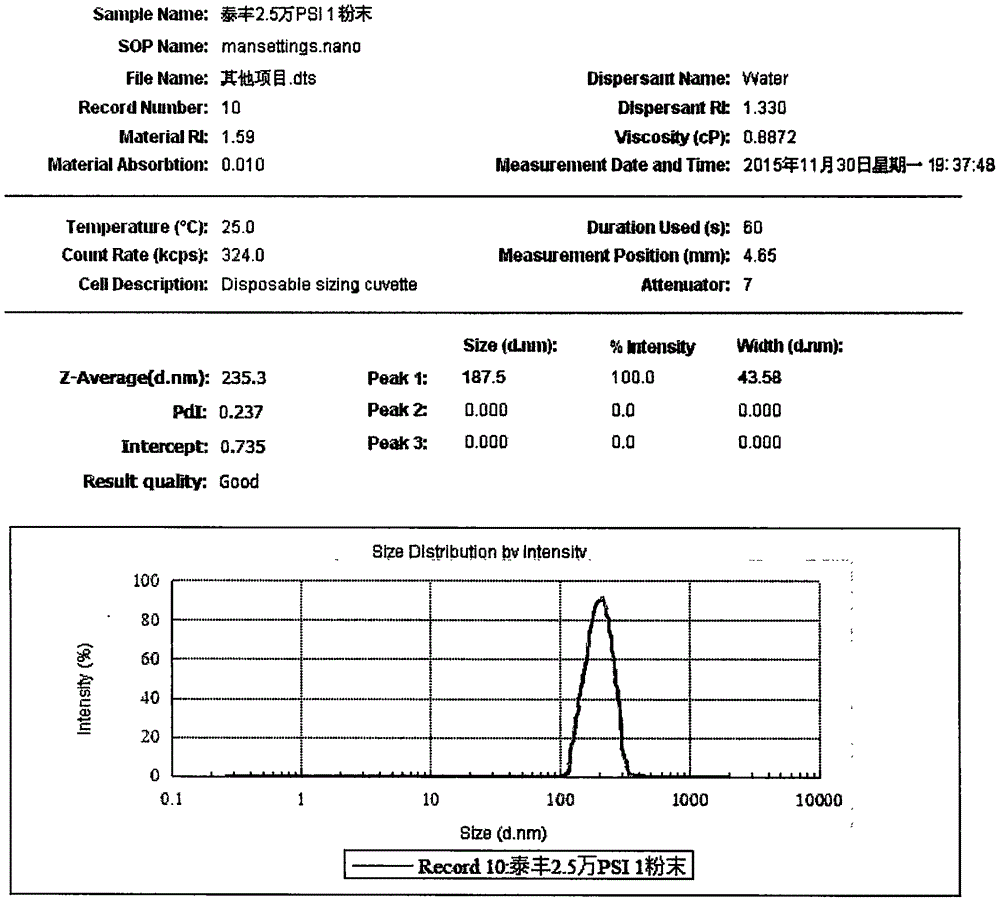
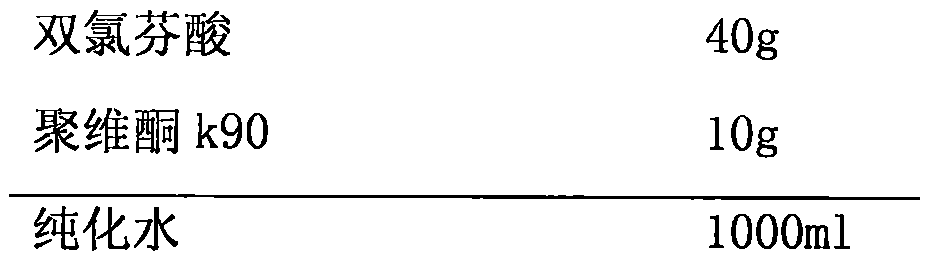
[0038] Suspension Prescription:
[0039]
[0040] Suspension Prescription:
[0041]
[0042] The preparation technology of low-dose diclofenac pharmaceutical composition comprises the following steps:
[0043] a) Weigh 10g of povidone k90, add 1000ml of purified water to prepare a 1% povidone k90 aqueous solution; weigh 40g of diclofenac, add it to a 1% povidone k90 aqueous solution, stir at 4000rpm for 30min, and prepare a uniformly dispersed Suspension: The suspension is homogenized using a D-6L high-pressure homogenizer, pretreated once under a pressure of 15000 psi; homogenized twice under a pressure of 23000 psi to obtain an emulsion;
[0044] b) The emulsion is spray-dried with a 6000Y spray dryer, the air inlet temperature is 55-60°C, the outlet air temperature is 50-55°C, the feed rate is 1000ml / h, the nozzle diameter is 0.7mm, and the pressure is 0.2Mpa to obtain dry powder;
[0045]c) Weigh 43.8g of dry powder, 8g of sodium carboxymethylcellulose, 4g of polox...
Embodiment 2
[0048] Suspension Prescription:
[0049]
[0050]
[0051] Prescription for granules:
[0052]
[0053] The preparation technology of low-dose diclofenac pharmaceutical composition comprises the following steps:
[0054] a) Weigh 10g of povidone k90, add 1000ml of purified water to prepare a 1% povidone k90 aqueous solution; weigh 40g of diclofenac, add it to a 1% povidone k90 aqueous solution, stir at 4000rpm for 30min, and prepare a uniformly dispersed Suspension: The suspension is homogenized using a D-6L high-pressure homogenizer, pretreated once under a pressure of 15000 psi; homogenized twice under a pressure of 23000 psi to obtain an emulsion;
[0055] b) The emulsion is spray-dried with a 6000Y spray dryer, the air inlet temperature is 55-60°C, the outlet air temperature is 50-55°C, the feed rate is 1000ml / h, the nozzle diameter is 0.7mm, and the pressure is 0.2Mpa to obtain dry powder;
[0056] c) Weigh 43.8g of dry powder, 354.1g of mannitol, 0.12g of sodi...
Embodiment 3
[0059] Suspension Prescription:
[0060]
[0061] Tablet prescription:
[0062]
[0063]
[0064] The preparation technology of low-dose diclofenac pharmaceutical composition comprises the following steps:
[0065] a) Weigh 10g of povidone k90, add 1000ml of purified water to prepare a 1% povidone k90 aqueous solution; weigh 40g of diclofenac, add it to a 1% povidone k90 aqueous solution, stir at 4000rpm for 30min, and prepare a uniformly dispersed Suspension: The suspension is homogenized using a D-6L high-pressure homogenizer, pretreated once under a pressure of 15000 psi; homogenized twice under a pressure of 23000 psi to obtain an emulsion;
[0066] b) The emulsion is spray-dried with a 6000Y spray dryer, the air inlet temperature is 55-60°C, the outlet air temperature is 50-55°C, the feed rate is 1000ml / h, the nozzle diameter is 0.7mm, and the pressure is 0.2Mpa to obtain dry powder;
[0067] c) Weigh 12g of sodium carboxymethylcellulose, add it to 100ml of pur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com