Protein co-loading chemotherapy drug and radiotherapy drug and application of protein
A chemotherapeutic drug and drug technology, applied in the field of medicine, can solve the problems of poor biocompatibility of nanocarriers, complicated material preparation process and high cost, achieve good water solubility and biocompatibility, simple preparation method, and improve hypoxia. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 Preparation of proteins co-loading chemotherapeutic and radiotherapy drugs 131 I-HSA-PTX
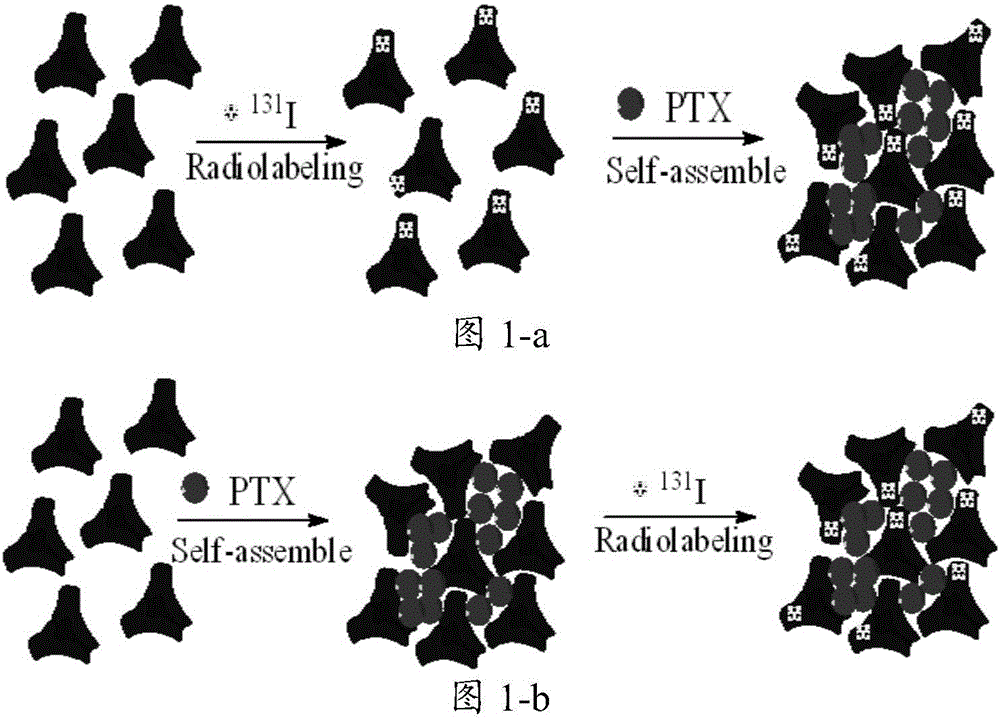
[0077] The preparation process is as figure 1 As shown in -a, albumin HSA is used as the substrate, and chemotherapy drugs and radionuclides are transferred.
[0078] Take 100 μCi of Na 131 I, add 1ml of 2mg / ml HSA aqueous solution, then add 30μL of 10mg / ml chloramine T solution (dissolved in PB buffer), shake and shake for 20 minutes, and use a 10kDa ultrafiltration tube to ultrafilter to remove untreated Reacted Na 131 I and chloramine T, to give 131 I labeled HSA solution. Then add 13 μL of 20 mg / ml PTX (dissolved in ethanol) (the amount of PTX added will change the size of the nanoparticles, the more the amount of PTX, the larger the particle size of the nanoparticles), stir overnight, and centrifuge at 14800 rpm for 5 Minutes to remove the precipitate, the product 131 I-HSA-PTX dispersed in the supernatant
Embodiment 2
[0079] Example 2 Preparation of proteins co-loading chemotherapeutic and radiotherapy drugs 131 I-HSA-PTX
[0080] The preparation process is as figure 1 As shown in -b, the albumin HSA is used as the substrate, and the chemotherapeutic drugs and radionuclides are transferred.
[0081] Add 13ul of 20mg / ml PTX (dissolved in ethanol) to 1ml of 2mg / ml HSA aqueous solution, stir overnight, centrifuge at 14800 rpm for 5 minutes to remove the precipitate, and the intermediate HSA-PTX is dispersed in the supernatant. Then add 100uCi of Na to the supernatant 131 The 10mg / ml chloramine T solution (dissolved in the PB damping fluid) of 1 and 30ul, shaking shakes 20 minutes, with the ultrafiltration tube ultrafiltration of 10KDa, remove unreacted Na 131 I and chloramine T to give the final product 131 I-HSA-PTX.
Embodiment 3131I
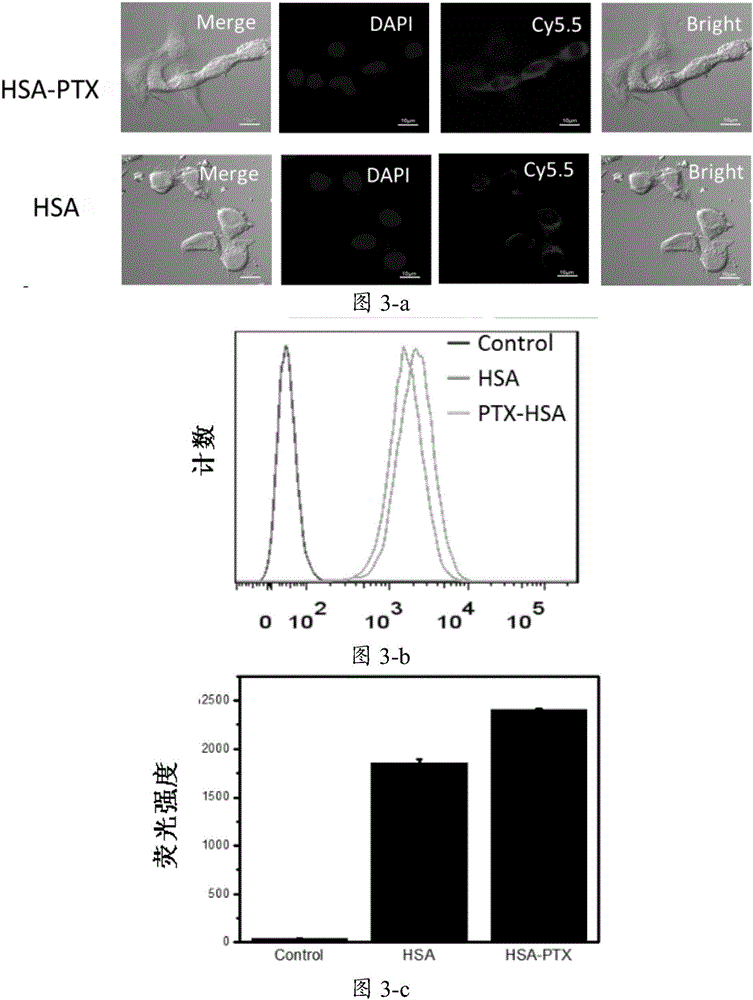
[0082] Example 3 131 Characterization of I-HSA-PTX
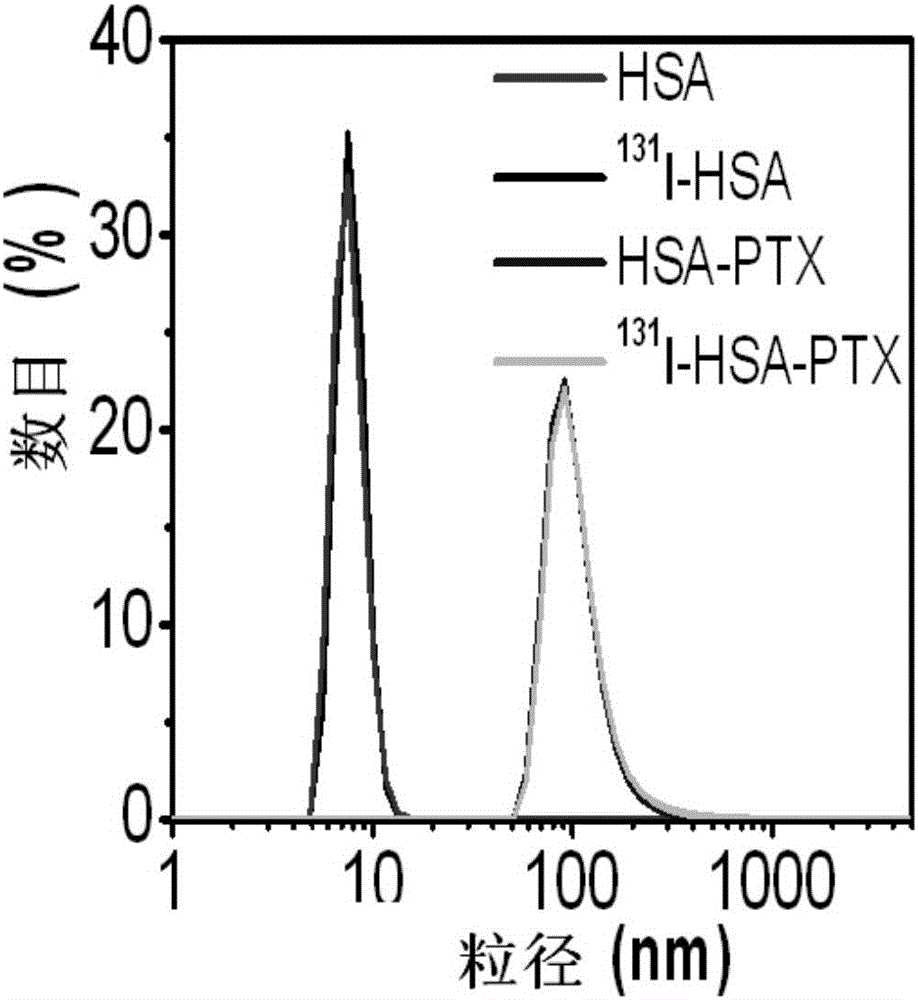
[0083] Made to embodiment 1 or 2 131I-HSA-PTX is characterized, embodiment 1 or 2 gained 131 The laser dynamic light scattering results of I-HSA-PTX are as follows figure 2 As shown, the hydration radius of pristine HSA and radiolabeled HSA is about 8nm, radiolabeling did not change the size of HSA; the intermediate HSA-PTX and the final product 131 The hydration radius of I-HSA-PTX is about 110nm, radiolabeling did not change the material size and loading of PTX.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com