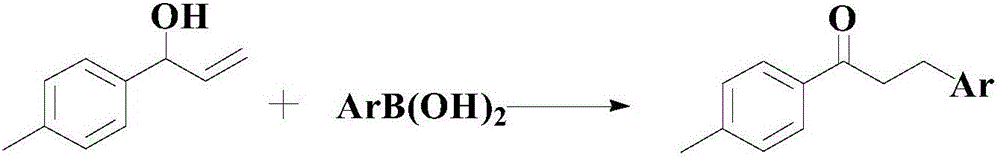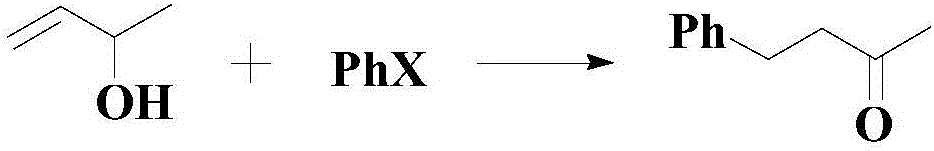Synthetic method of medical intermediate diaryl ketone compound
A synthesis method and a technology for diaryl ketones are applied in the synthesis of ketone compounds and in the field of synthesis of diaryl ketone compounds, which can solve the problems such as the yield needs to be improved, the substrate needs to be expanded, etc., to achieve the expansion of material sources and high efficiency Preparation, the effect of broad industrial prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
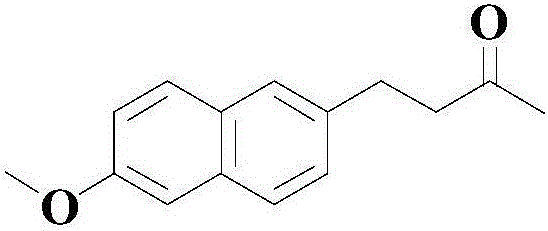
[0042]
[0043] At room temperature, add 100 mmol of the above formula (I) to an appropriate amount of organic solvent (a mixture of N,N-dimethylformamide (DMF) and polyethylene glycol 200 (PEG-200) at a volume ratio of 4:1) Compound, 40mmol formula (II) compound, 11mmol catalyst bis(triphenylphosphine)cyclopentadienyl ruthenium chloride, 100mmol oxidant bis(trifluoroacetic acid) iodobenzene (PhI(TFA) 2), 20mmol organic ligand L1 and 60mmol base, 5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), then heated to 70°C under stirring, and stirred at this temperature for reaction 12 Hour;
[0044] After the reaction was completed, the reaction system was naturally cooled to room temperature, and the pH value was adjusted to be neutral, filtered, the filtrate was washed with saturated brine, and then extracted with ethyl acetate for 2-3 times, the organic phases were combined, dried over anhydrous sodium sulfate, Concentrated under reduced pressure, the residue was subjected to silica ge...
Embodiment 2
[0047]
[0048] At room temperature, add 100 mmol of the above formula (I) to an appropriate amount of organic solvent (a mixture of N,N-dimethylformamide (DMF) and polyethylene glycol 200 (PEG-200) at a volume ratio of 4:1) Compound, 80mmol formula (II) compound, 7mmol catalyst bis(triphenylphosphine) cyclopentadienyl ruthenium chloride, 150mmol oxidant bis(trifluoroacetic acid) iodobenzene (PhI(TFA) 2 ), 10mmol organic ligand L1 and 100mmol base, 5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), then heated to 100°C under stirring, and stirred at this temperature for 8 Hour;
[0049] After the reaction was completed, the reaction system was naturally cooled to room temperature, and the pH value was adjusted to be neutral, filtered, the filtrate was washed with saturated brine, and then extracted with ethyl acetate for 2-3 times, the organic phases were combined, dried over anhydrous sodium sulfate, Concentrate under reduced pressure, and the residue is subjected to silica gel col...
Embodiment 3
[0052]
[0053] At room temperature, add 100 mmol of the above formula (I) to an appropriate amount of organic solvent (a mixture of N,N-dimethylformamide (DMF) and polyethylene glycol 200 (PEG-200) at a volume ratio of 4:1) Compound, 60mmol formula (II) compound, 8mmol catalyst bis(triphenylphosphine) cyclopentadienyl ruthenium chloride, 120mmol oxidant bis(trifluoroacetic acid) iodobenzene (PhI(TFA) 2 ), 15mmol organic ligand L1 and 80mmol base, 5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), then heated to 80°C under stirring, and stirred at this temperature for reaction 11 Hour;
[0054] After the reaction was completed, the reaction system was naturally cooled to room temperature, and the pH value was adjusted to be neutral, filtered, the filtrate was washed with saturated brine, and then extracted with ethyl acetate for 2-3 times, the organic phases were combined, dried over anhydrous sodium sulfate, Concentrate under reduced pressure, and the residue is subjected to silica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com