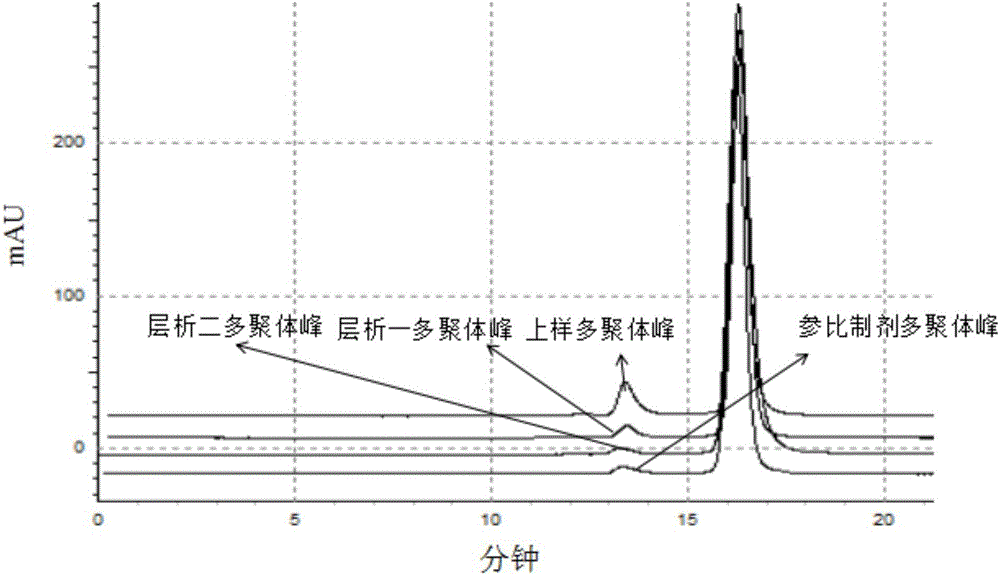
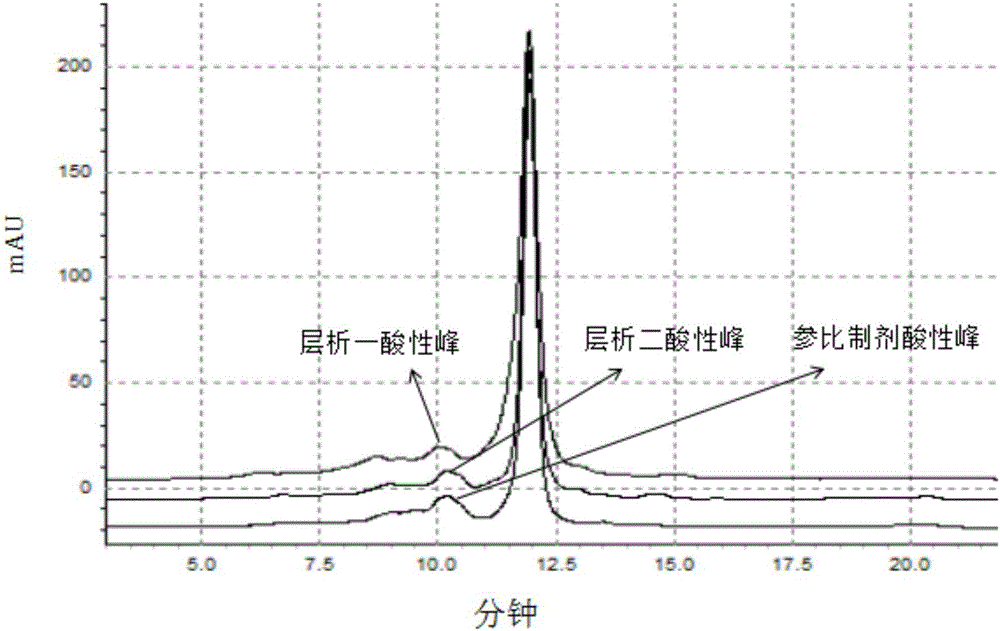
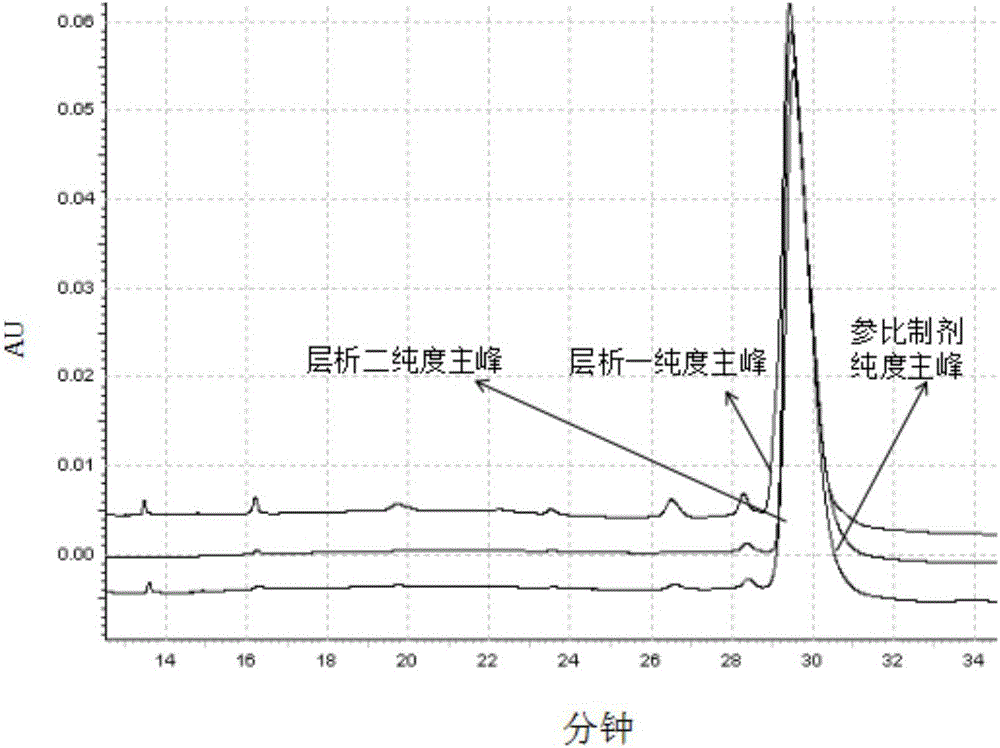
Purification method of anti-VEGF (Vascular Endothelial Growth Facto) type monoclonal antibody
A technology of monoclonal antibody and purification method, which is applied in the field of purification of anti-VEGF monoclonal antibody, and can solve problems such as high economic cost, low recovery rate, and protein A ligand drop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1) Clarification of cell fluid
[0052] Using an eppendorf centrifuge, centrifuge the CHO cell culture supernatant twice at 10,000 g to remove cells and cell debris, pass through a 0.2 μm filter membrane to further reduce the turbidity of the sample, and adjust the pH to 7.0, which is the monoclonal antibody composition to be loaded.
[0053] 2) Composite chromatography
[0054] Using an AKTA purifier-100 (GE Healthcare) chromatography system, 9.4 mL of MEP (PALL Company) composite chromatography medium was loaded on a PALL chromatography column (1.0*20 cm, PALL Company). Fully equilibrate the recombination chromatography column with equilibration buffer, wait until the UV absorption at 280nm returns to the baseline, and start loading the sample when the conductivity and pH remain stable, wash the unbound fully integrated protein with the first washing buffer, and wait until the UV absorption at 280nm After the absorption returns to the baseline, and the conductivity a...
Embodiment 2
[0098] 1) Clarification of cell fluid
[0099] Using an eppendorf centrifuge, centrifuge the CHO cell culture supernatant twice at 10,000 g to remove cells and cell debris, pass through a 0.2 μm filter membrane to further reduce the turbidity of the sample, and adjust the pH to 6.5, which is the monoclonal antibody composition to be loaded.
[0100] 2) Composite chromatography
[0101] Using the AKTA purifier-100 (GE Healthcare) chromatographic system, 1.2 mL of MEP (PALL Company) composite chromatographic medium was loaded on a Tricorn 10 / 20 (GE Company) chromatographic column. Fully equilibrate the recombination chromatography column with equilibration buffer (50mM PBS pH7. 0) Wash the unbound whole protein, wait until the UV absorption at 280nm returns to the baseline, and after the conductivity and pH remain stable, then wash with the second buffer solution (50mM PBS pH6.0), and wait until the UV absorption at 280nm returns to the baseline Pure, and after the conductivit...
Embodiment 3
[0111] 1) Clarification of cell fluid
[0112] Using an eppendorf centrifuge, centrifuge the CHO cell culture supernatant twice at 10,000 g to remove cells and cell debris, pass through a 0.2 μm filter membrane to further reduce the turbidity of the sample, and adjust the pH to 6.8, which is the monoclonal antibody composition to be loaded.
[0113] 2) Composite chromatography
[0114] Using the AKTA purifier-100 (GE Healthcare) chromatographic system, 1.2 mL of MEP (PALL Company) composite chromatographic medium was loaded on a Tricorn 10 / 20 (GE Company) chromatographic column. Fully equilibrate the recombination chromatography column with equilibration buffer (50mM PBS pH7. 0) Wash the unbound whole protein, wait until the UV absorption at 280nm returns to the baseline, and after the conductivity and pH remain stable, then wash with the second buffer solution (50mM PBS pH6.0), and wait until the UV absorption at 280nm returns to After the base is pure, and the conductivity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com