ADMA (Asymmetric Dimethylarginine) immunoassay reagent and detection method
A reagent, glycine technology, applied in the field of medical immunology in vitro diagnosis, to achieve the effect of high specificity, high immunogenicity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
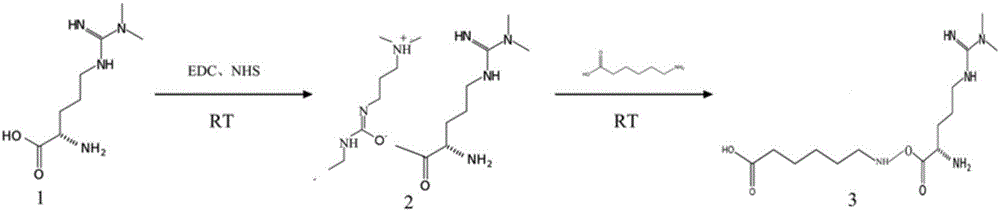
[0029] Example 1: Synthesis and structure confirmation of ADMA derivative 1
[0030] The biochemical structure of the ADMA derivative 1 used in the following examples is as figure 2 Shown in compound 3 in, the synthetic route is as follows figure 2 .
[0031] Concrete synthetic steps:
[0032] 1) Weigh 900mg of compound 1 (ADMA) and dissolve it in 90ml of pyridine, immediately add 360 mg of EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) and 540mg of NHS (N-hydroxysuccinimide), and put the solution at room temperature Under stirring for 30 hours, the synthetic solution of compound 2 was obtained;
[0033] 2) Add 126ul of β-mercaptoethanol to the above solution, stir at room temperature for 30min, inactivate excess EDC, and desalt into PBS with pH 7.2;
[0034] 3) Dissolve 15.00g of aminocaproic acid in 10mL of PBS solution with pH 7.2, take 50mL of the above synthetic solution and slowly add it dropwise to the solution of aminocaproic acid, stir at roo...
Embodiment 2
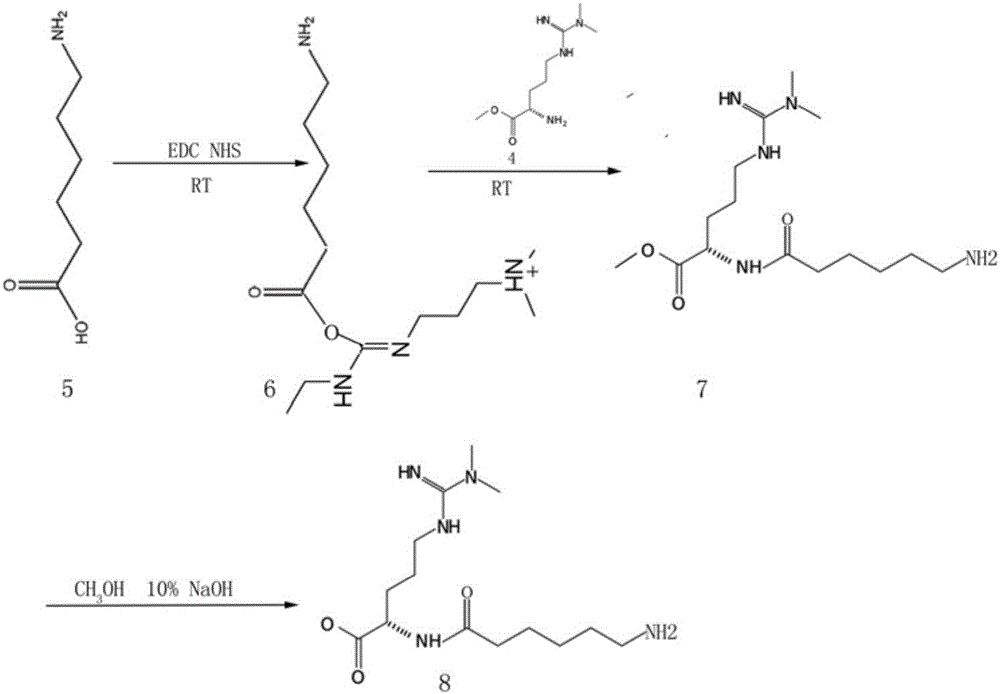
[0037] Example 2: Synthesis and structure confirmation of ADMA derivative 2
[0038] The biochemical structure of the ADMA derivative 2 used in the following examples is as image 3 Shown in compound 8, its synthetic route is as follows image 3 .
[0039] Concrete synthetic route is as follows:
[0040] 1) Weigh 100mg of ADMA and dissolve it in concentrated sulfuric acid, add 1200mg of methanol, and heat to reflux for 2h. After the synthetic solution was diluted with water, the solvent was evaporated by a reduced pressure method, and finally a white solid compound 4 was obtained;
[0041] 2) Weigh 500mg of compound 5 (aminocaproic acid) and dissolve it in 50ml of pyridine, immediately add 400mg of EDC (1-ethyl-3-3-dimethylaminopropyl]carbodiimide hydrochloride) and 600mg of NHS (N-hydroxysuccinimide), and put the solution at room temperature Under stirring for 30 hours, the synthetic solution of compound 6 was obtained;
[0042] 3) Add 90ul of β-mercaptoethanol to the ab...
Embodiment 3
[0048] Example 3: ADMA immunogen synthesis
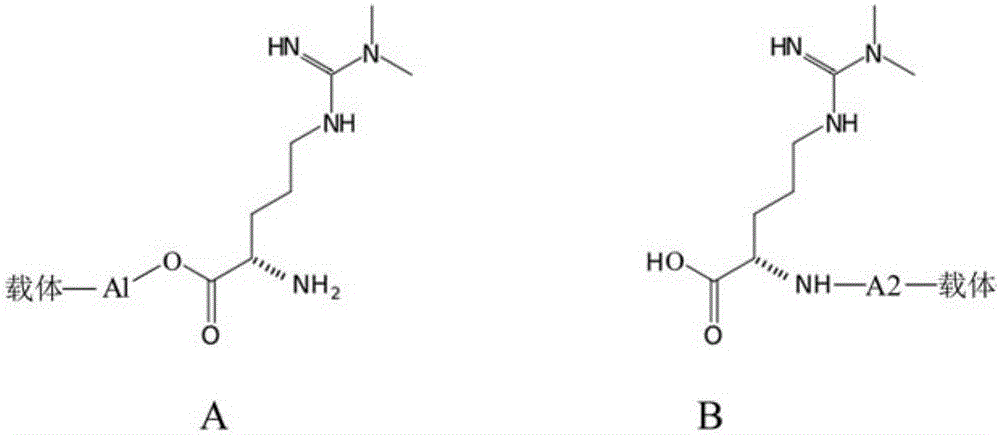
[0049] ADMA immunogen is composed of rabbit albumin or mouse albumin with figure 1 The ADMA derivatives shown are connected by linking groups. In this embodiment, the synthesis method of the immunogen is described in detail by taking the ADMA derivative whose linking arm is aminocaproic acid as an example.
[0050] Rabbit albumin-ADMA carboxyl derivative A1 immunogen synthesis:
[0051] Specific steps are as follows:
[0052] 1) Dissolve 40mg of rabbit albumin in 4mL of 0.1M MES buffer at pH 5.0, and place the above solution in beaker A;
[0053] 2) Dissolve 30mg of ADMA derivative 1 in 6mL of 0.1M MES buffer at pH 5.0 and add to beaker A;
[0054] 3) Add 50 mg of EDC to beaker A to obtain a mixed solution, stir and react at room temperature for 2 hours; dialyze and purify the stirred mixed solution with neutral PBS buffer to obtain rabbit albumin-ADMA immunogen, which is stored at -70°C .
[0055] Synthesis of murine albumin-A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com