Dipeptide derivative for detecting angiotensin I as well as preparation method and application thereof
An anti-angiotensin and derivative technology, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of low detection sensitivity, poor specificity, and inability to detect, achieve high immunogenicity, high accuracy, and achieve full automated effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
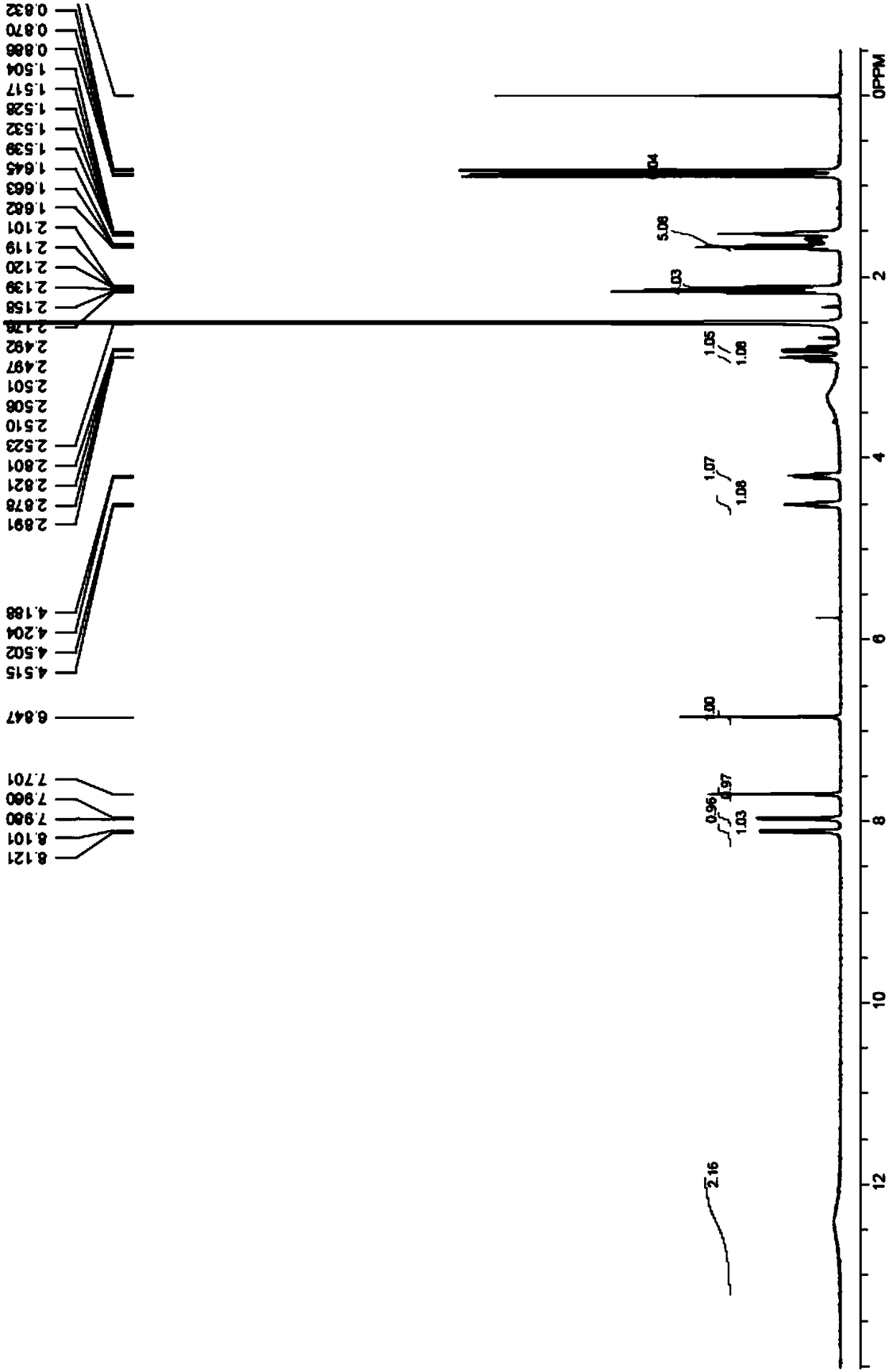
[0055] Embodiment 1: the synthesis of leucine-histidine dipeptide derivative
[0056] The chemical structure of the leucine-histidine dipeptide derivative is shown in formula (I):
[0057]
[0058] The synthetic route and preparation steps of the above-mentioned leucine-histidine dipeptide derivatives are as follows:
[0059]
[0060] Concrete synthetic steps are as follows:
[0061] (1) Synthesis of compound 2
[0062] Weigh 25g (86.2mmol) of compound 1 and dissolve it in 250mL of dichloromethane (DCM), then add 17.2g (103.5mmol) of compound A, 26.5g (172.4mmol) of 1-hydroxybenzotriazole (HOBT ), 38g (172.4mmol) of carbodiimide (EDCI), and 50g (500mmol) of triethanolamine (TEA) were prepared as reaction mixture solution I. The reaction mixture I was stirred overnight at 30°C. After the reaction, an appropriate amount of purified water was added to the reaction mixture solution I, and then 150 mL of DCM was used for extraction, and the extraction step was repeated 4 ...
Embodiment 2
[0074] Example 2: Synthesis of Leucine-Histidine Dipeptide Immunogen
[0075] The leucine-histidine dipeptide immunogen is composed of bovine serum albumin (Bovine Serum Albumin, BSA) and -CO-(CH 2 ) 3 The -CO- group is connected, and the structural formula is shown in the following formula (II):
[0076]
[0077] The concrete steps of the synthetic method of this immunogen are as follows:
[0078] 1. Dissolve 200mg bovine serum albumin in 50ml 0.2M, pH 8.5 phosphate buffer;
[0079] 2. Add the following chemicals into a small beaker and stir to dissolve: 200mg of the leucine-histidine dipeptide derivative synthesized in Example 1, 3.5ml of dimethylformamide, 3.5ml of ethanol, 7.0ml of 10mM, pH 5.0 Potassium phosphate buffer solution, 200mg 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide, 50mg N-hydroxyl sulfosuccinimide, these chemicals were stirred and dissolved at room temperature for 30min ;
[0080] 3. Add the dissolved solution dropwise to the BSA solution, and s...
Embodiment 3
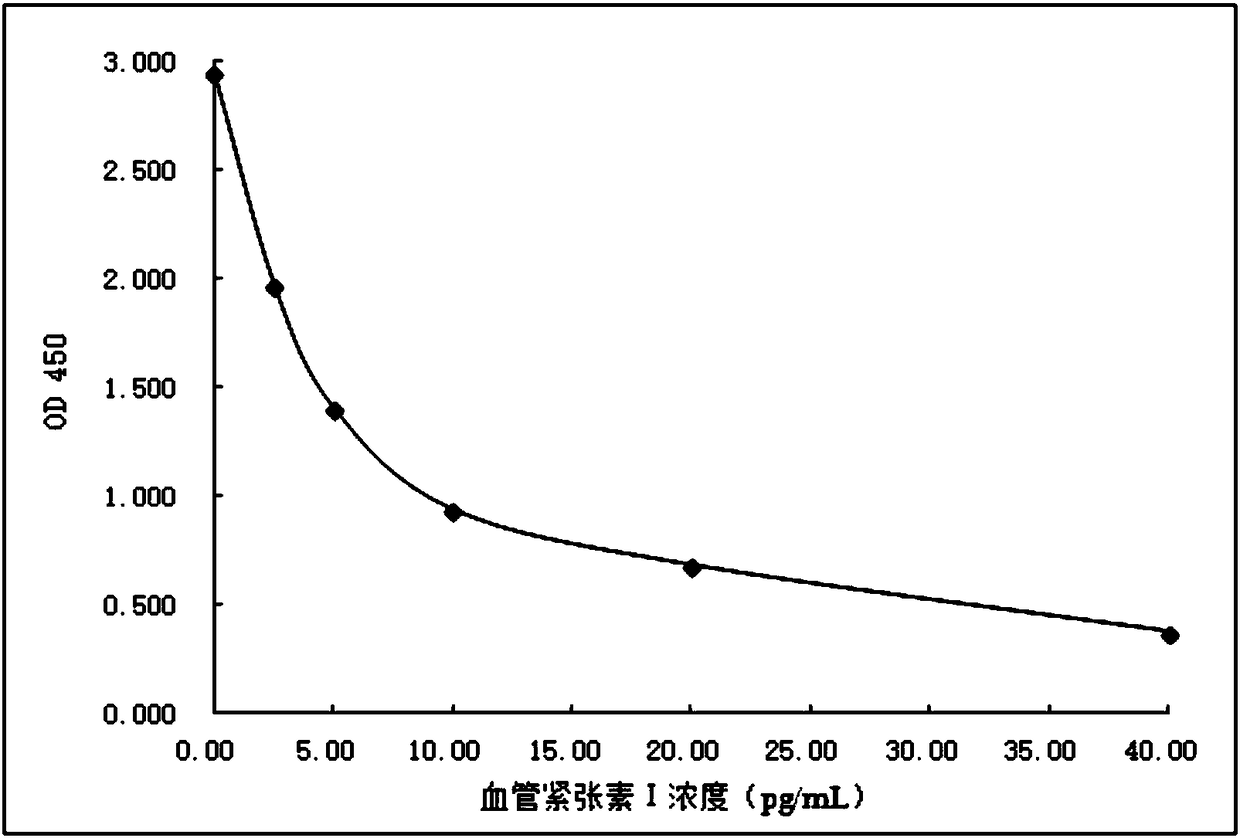
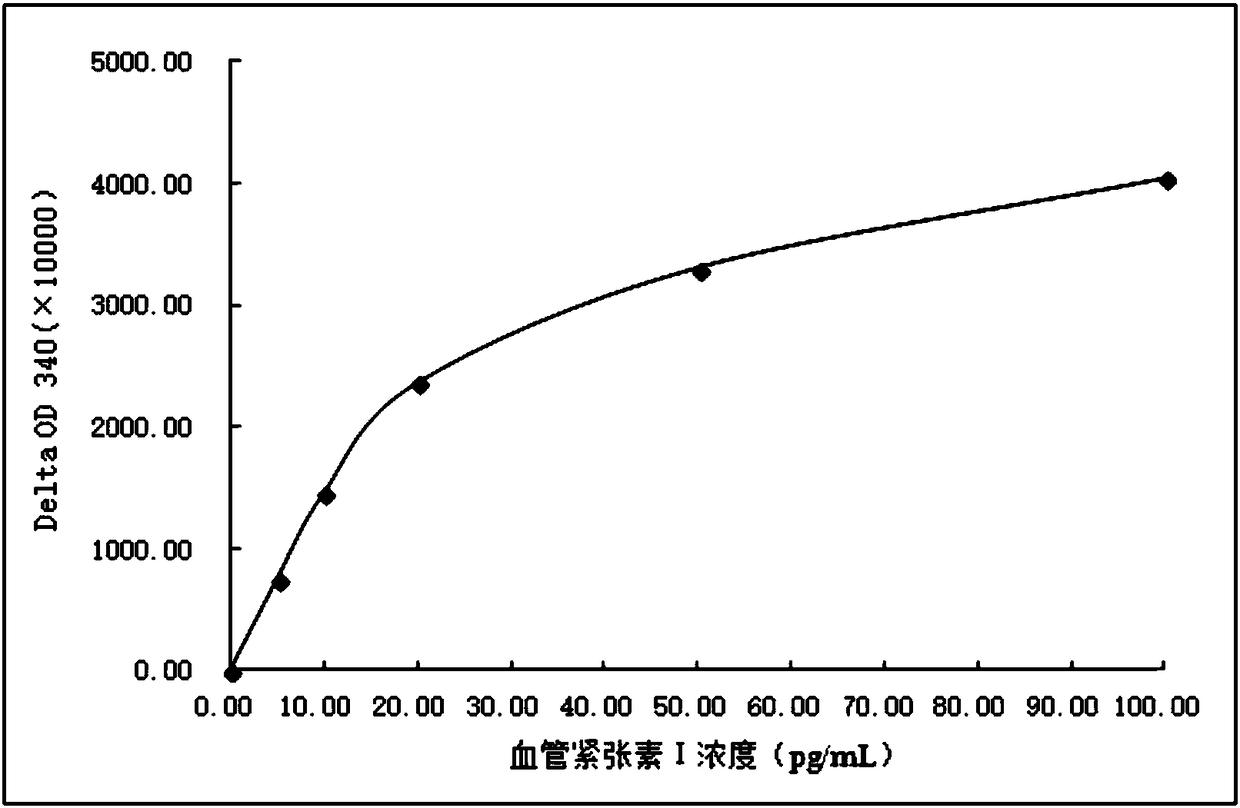
[0082] Example 3: Preparation of Anti-Angiotensin I Specific Antibody
[0083] The leucine-histidine dipeptide immunogen prepared in Example 2 was inoculated into experimental animal rabbits by conventional methods, and the antiserum was taken after booster immunization. The specific steps were as follows:
[0084] 1. Dilute the above synthesized leucine-histidine dipeptide immunogen to 1.0 mg / ml with PBS to obtain an antigen solution, then mix 1.0 ml of the antigen solution with Freund's complete adjuvant, and inject the experimental animal rabbit .
[0085] 2. After 2 to 3 weeks, inject 1.0ml of the same antigen solution and incomplete Freund's adjuvant to the above-mentioned experimental rabbit once, and then inject once every four weeks, a total of 4 injections.
[0086] 3. Take blood from the experimental animal rabbit in step 2, separate and purify to obtain anti-angiotensin I specific antibody with a titer of 1:30000-1:50000.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com