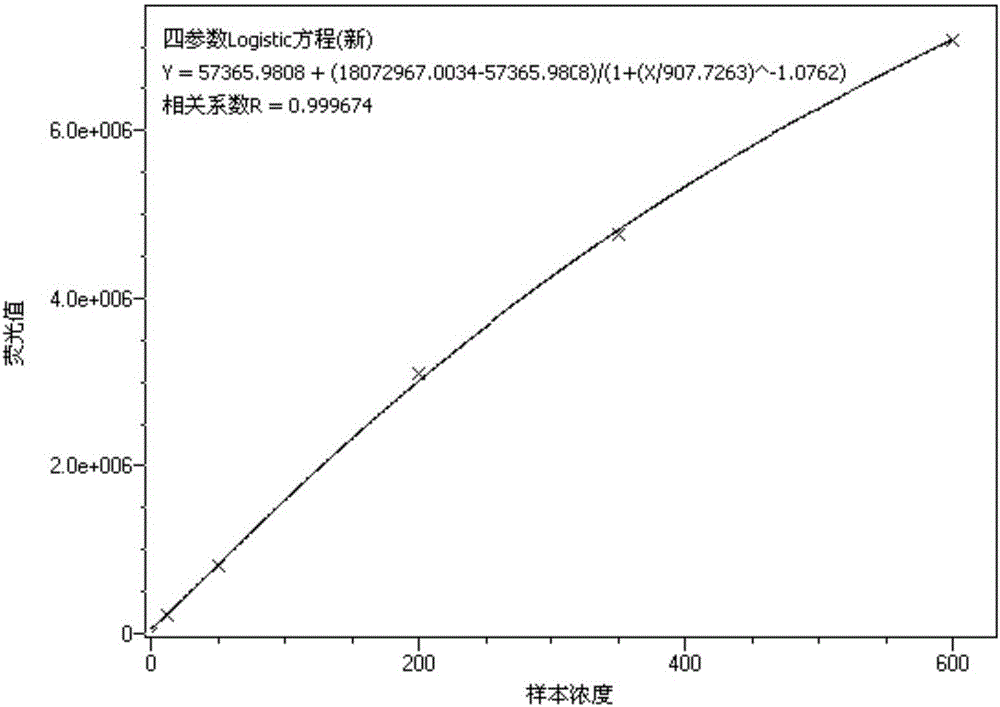
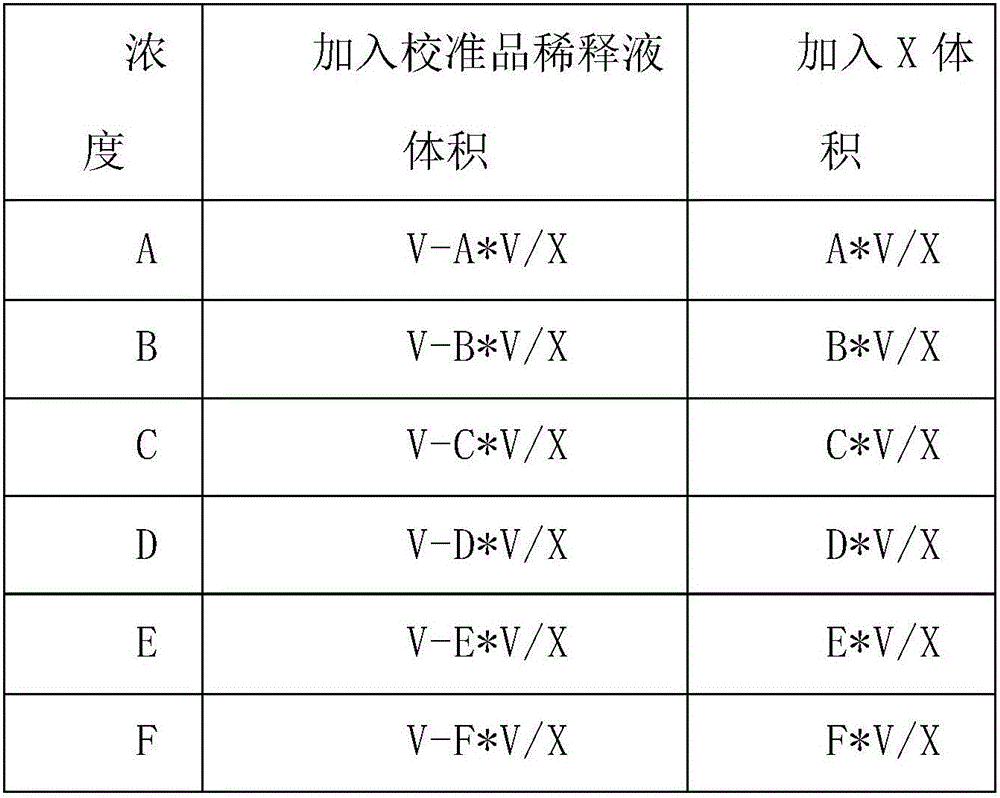
Whole range C-reactive protein test kit and detection method thereof
A reactive protein and kit technology, applied in biological tests, measuring devices, material inspection products, etc., can solve the problems of timely diagnosis of unwell emergency and clinical patients, long time for C-reactive protein detection, and complicated operation, and achieves reduction of non-specific The effect of heterosexual adsorption, high affinity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0056] 1. Operating procedures for preparing magnetic bead buffer: Take the preparation of 1L as an example:
[0057] 1. According to the preparation amount, select a suitable container and add 0.7kg / 700ml of pure water;
[0058] 2. Weigh 6.02g of Tris and 0.99g of NaN3 in a container, mix well, and stir at room temperature for 2 hours;
[0059] 3. Check the pH of the solution, it should be around 10;
[0060] 4. Adjust the pH value to 8.0;
[0061] 5. Measure 2.76ml of Tween-20; weigh 0.99g of neomycin sulfate; 0.03g of tetracycline; 4.97g of BSA in a container, stir for 1 hour, and adjust the pH value to 8.0±0.05;
[0062] 6. Set the volume to 1L, adjust the pH value to 8.0±0.05;
[0063] 7. Filter with a 0.22um filter and store at 4°C.
[0064] 2. Preparation process of magnetic particle reagent
[0065] Activate ferric oxide microspheres with a diameter of 0.1 micron with glutaraldehyde, mix at room temperature for 4 hours, wash with 0.01mol / L PBS pH7.4 buffer solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luster | aaaaa | aaaaa |
| correlation coefficient | aaaaa | aaaaa |
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com