Flavones-6-hydroxylase and application thereof to scutellarin synthesis
A kind of hydroxylase and flavonoid technology, which is applied to flavonoid-6-hydroxylase and its application field in the synthesis of scutellarin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0128] The preparation method of scutellarin
[0129] The present invention also provides a preparation method of scutellarin, comprising the steps of:
[0130] (i) in the presence of cytochrome reductase (CPR), utilize flavone-6-hydroxylase to catalyze apigenin, thereby obtaining scutellarein;
[0131] with
[0132] (ii) performing a glycosylation reaction on the scutellarein obtained in step (i), thereby obtaining scutellarin;
[0133]
[0134] The main advantages of the present invention include:
[0135] (1) The present invention finds for the first time that flavone-6-hydroxylase can catalyze the hydroxylation of C6 of apigenin, thereby obtaining scutellarein.
[0136] (2) The present invention provides for the first time flavone-6-hydroxylase derived from scutellaria breviscapus, and finds that the flavone-6-hydroxylase derived from scutellaria breviscapus can catalyze the C6 of apigenin to carry out the catalytic reaction of hydroxylation, thereby obtaining wild...
Embodiment 1
[0142] Example 1 Obtaining of EbF6H Encoding Nucleotides
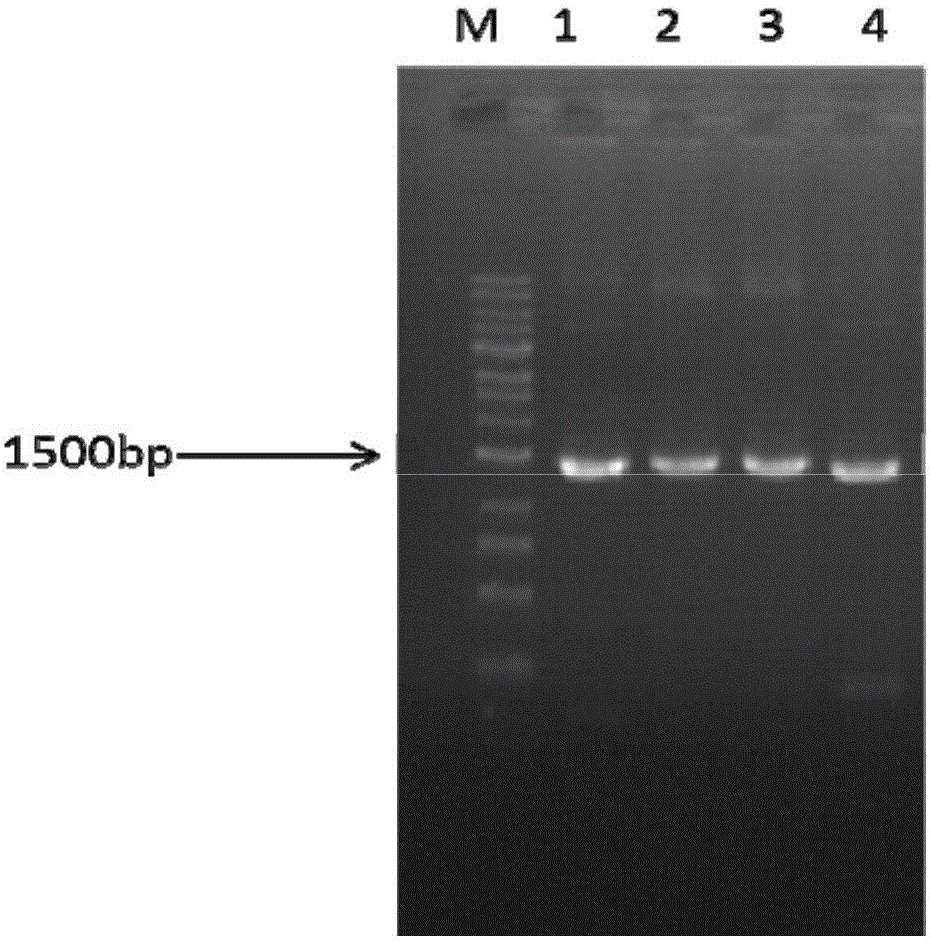
[0143] According to the nucleotide sequence information (SEQ ID NO.: 2) provided by the present invention, it is obtained by amplifying or isolating the RNA reverse transcriptome of Breviscapus breviscapus. Specifically, RNA can be extracted from plants using conventional methods or using the Easy spin plus plant RNA rapid extraction kit (purchased from Beijing Aidelai Biotechnology Co., Ltd.), and then the obtained RNA is used as a template to synthesize cDNA using First Strand The kit (Thermo, USA) reverse-transcribed the cDNA obtained according to the operation steps on the kit, and used it as a template, using primers EbF6H-F1 (SEQ ID NO: 9) and EbF6H-R1 (SEQ ID NO: 10) using conventional The PCR method specifically amplifies the coding nucleotide of EbF6H. PCR amplified fragments were subjected to agarose gel electrophoresis, the results were as follows figure 2 shown.
Embodiment 2
[0144] The acquisition of the coding nucleotide of embodiment 2 CcsF6H
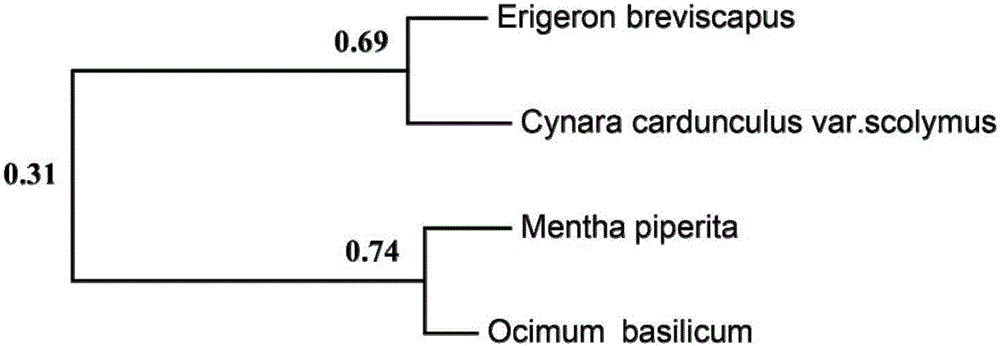
[0145] Using the amino acid sequence of EbF6H of Erigeron breviscapus to compare with other Asteraceae species, it is found that there is an amino acid sequence with about 69% similarity with EbF6H in Artichoke, another Asteraceae species whose genome has been sequenced, and its Genbank number is KVH90146.1. After identification, it was deduced that this gene also has a similar function of flavonoid 6-hydroxylation, and it was named CcsF6H.
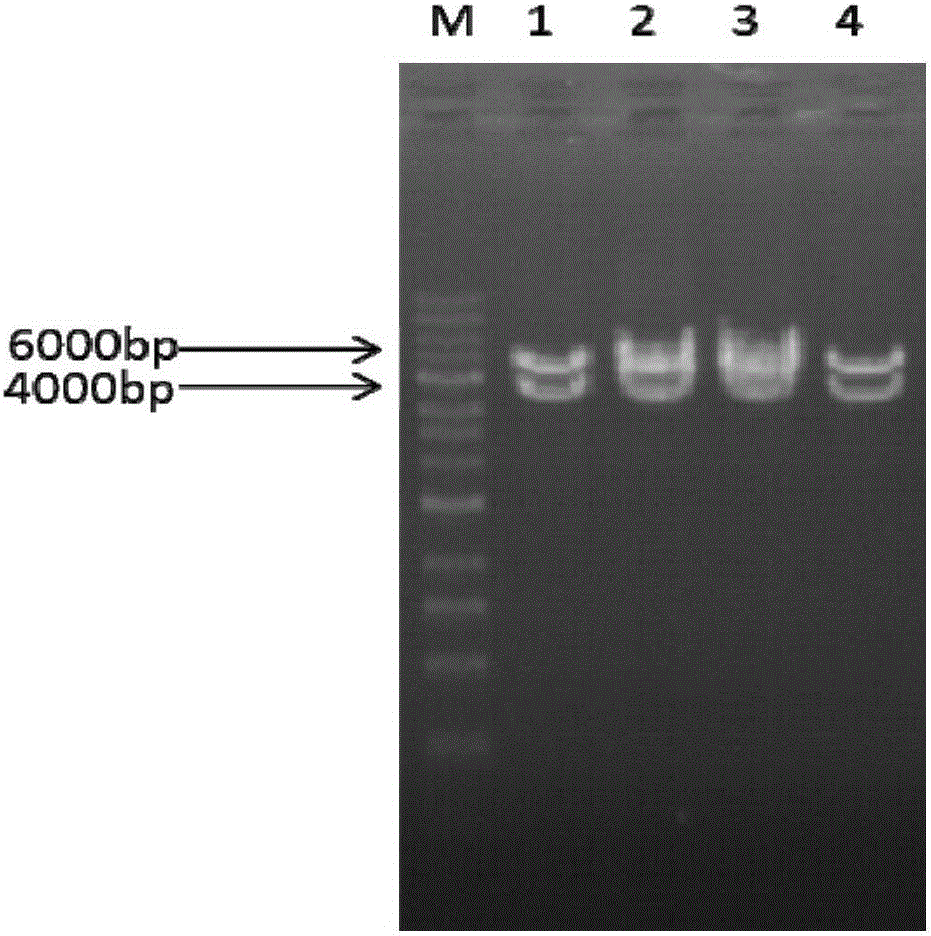
[0146] According to the amino acid sequence of SEQ ID NO: 3, the nucleotide sequence codon is optimized for the target host cell (Saccharomyces cerevisiae) and artificially synthesized to obtain the sequence shown in SEQ ID NO: 6. Using CcsF6H-F1 (SEQ ID NO: 11) and CcsF6H-R1 (SEQ ID NO: 12) as primers, a conventional PCR method was used to specifically amplify the coding nucleotide of CcsF6H. PCR amplified fragments were subjected to agarose gel electrophoresis, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com