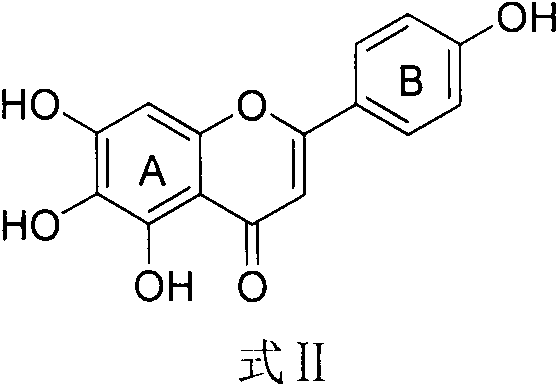
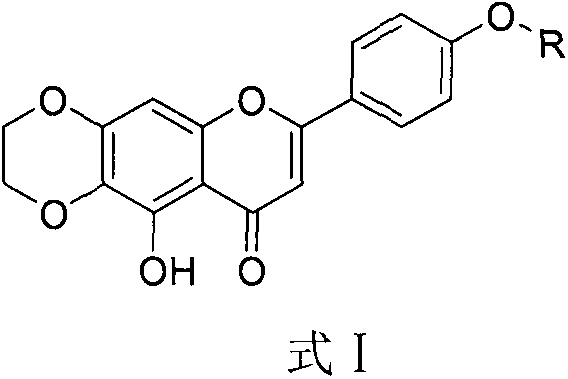
Scutellarein derivative and preparation method and application thereof
A technology of scutellarin and derivatives, applied in the field of scutellarin derivatives and their preparation, can solve the problems of low bioavailability, limited preparation and clinical application, fast metabolism in vivo and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
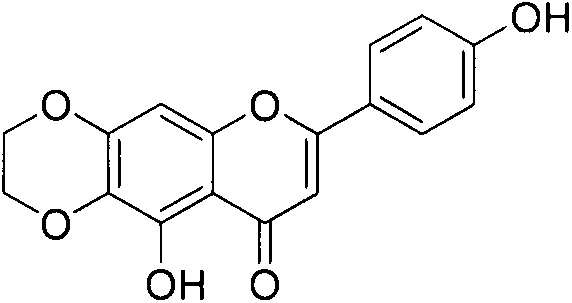
[0045] Example 1 10-Hydroxy-7-(4'-hydroxyphenyl)-2,3-dihydro-9H-{[1,4]-dioxo[2,3-g]benzo}pyran- Preparation of 9-keto (compound 2)
[0046] Dissolve scutellarein (286.24mg, 1mmol) in 5mL of anhydrous DMF, add potassium carbonate (207.3mg, 1.5mmol) and dibromoethane (129.87μL, 1.5mmol) at room temperature, N 2 Under protection, react at 110°C for 2h. After the reaction, the reaction liquid was poured into 40 mL of water, a large amount of yellow solids were precipitated, filtered with suction, and the filter cake was recrystallized with methanol to obtain 90 mg of yellow powder with a yield of 29%. Identified as 10-hydroxy-7-(4'-hydroxyphenyl)-2,3-dihydro-9H-{[1,4]-dioxo[2,3-g]benzo}pyran- 9-keto.
[0047]
[0048] ESI-MS m / z: 335.1[M+Na] + , 313.1[M+H] + , 311.1[M-H] - , with a relative molecular mass of 312.
[0049] 1 H-NMR (300MHz, DMSO-d 6 )δ13.03(s, 1H, 10-OH), 10.39(s, 1H, 4'-OH), 7.93(d, 2H, J=8.6Hz, 2', 6'-OH), 6.91(d, 2H, J=8.6Hz, 3', 5'-OH), 6.81(s, 1H, ...
Embodiment 2
[0050] Example 2 10-Hydroxy-7-(4'-epoxypropylphenyl)-2,3-dihydro-9H-{[1,4]-dioxo[2,3-g]benzo} Preparation of pyran-9-one (compound 3)
[0051] Compound 2 (120.00mg, 0.4mmol) was dissolved in 4mL DMF, and potassium carbonate (110.56mg, 0.8mmol) and potassium iodide (66.40mg, 0.4mmol) were added sequentially, and chlorinated propylene oxide (37.63μL, 0.48mmol), reacted at 85°C for 5h. After the reaction was completed, it was cooled to room temperature, and the reaction solution was extracted with 40 mL of ethyl acetate, washed 3 times with saturated brine, 20 mL each time, the organic layer was dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The product was separated and purified by silica gel column chromatography, washed with The stripping agent was dichloromethane to obtain 56.00 mg of yellow powder with a yield of 38.00%. Identified as 10-hydroxy-7-(4'-epoxypropylphenyl)-2,3-dihydro-9H-{[1,4]-dioxo[2,3-g]benzo} Pyran-9-one.
[...
Embodiment 3
[0055] Example 3 10-Hydroxy-7-(4'-(2-oxo-2-phenylethoxy)phenyl)-2,3-dihydro-9H-{[1,4]-dioxo Preparation of [2,3-g]benzo}pyran-9-one (compound 4)
[0056] Compound 2 (80.00mg, 0.26mmol) was dissolved in 3mL of anhydrous DMF, potassium carbonate (70.87mg, 0.51mmol) and potassium iodide (43.16mg, 0.26mmol) were added sequentially, and 2-bromoacetophenone was added after reacting at room temperature for 30min (61.37mg, 0.31mmol), react at room temperature for 12h. After the reaction was completed, the reaction liquid was extracted with 40 mL of ethyl acetate, washed with saturated brine three times, 20 mL each time, the organic layer was dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The product was separated and purified by silica gel column chromatography, and the eluent Petroleum ether-dichloromethane (1:20) to obtain 56.00 mg of a light yellow powder with a yield of 50.45%. Identified as 10-hydroxy-7-(4'-(2-oxo-2-phenylethoxy)phen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com