Functional cyclic olefin copolymer and preparation method thereof
A cyclic olefin copolymer, functional technology, applied in the field of functional cyclic olefin copolymer and its preparation, can solve the problems of low reactivity, low access rate of polar groups, and inability to control the content of polar groups and other problems to achieve the effect of ensuring high activity and strong activity copolymerization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
[0051] A kind of preparation method of functional cyclic olefin copolymer, concrete preparation steps are:
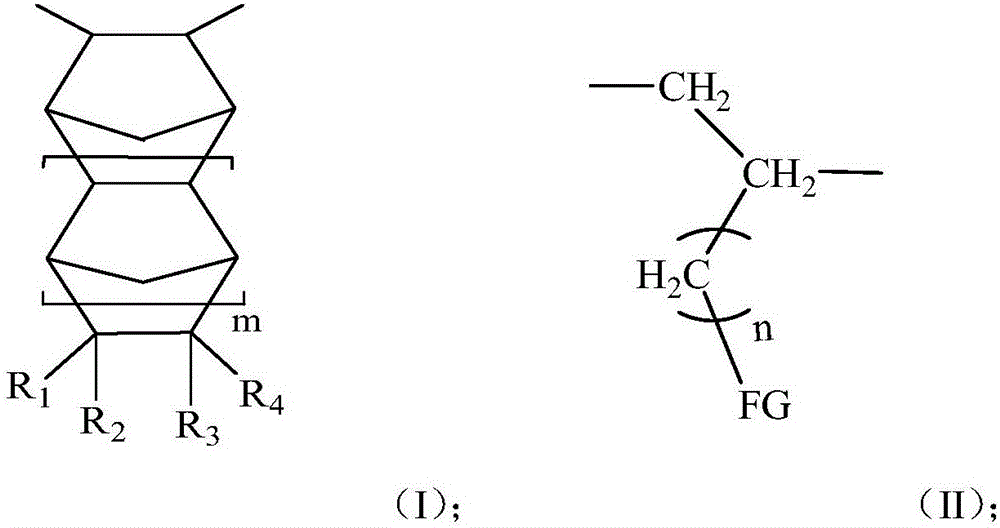
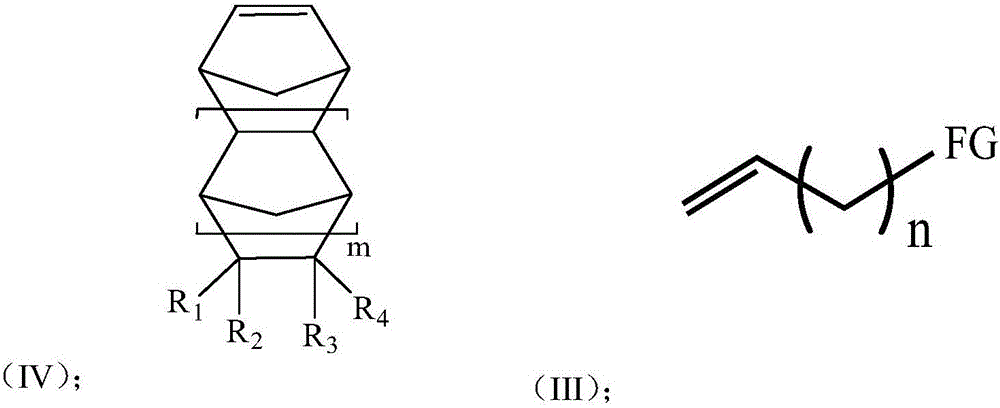
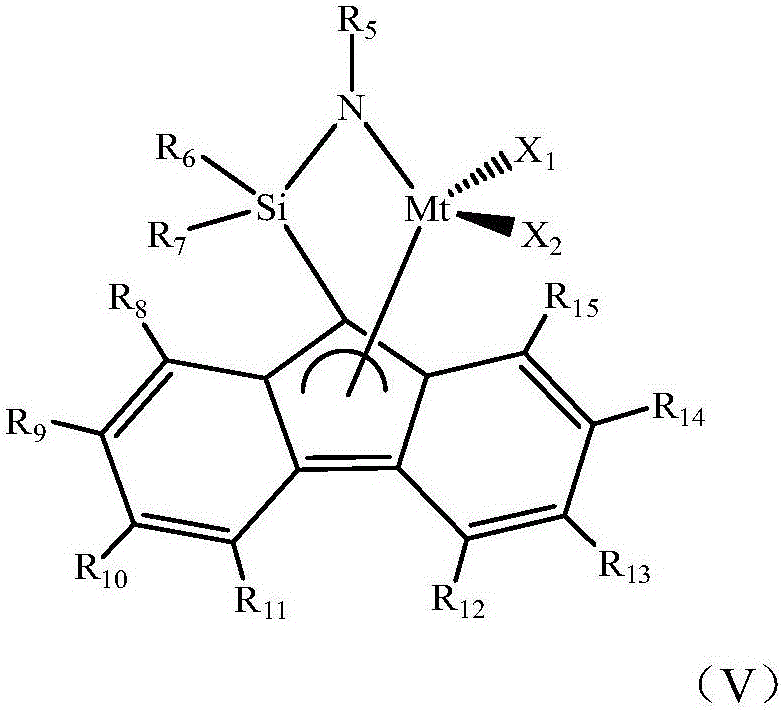
[0052] 1) Under a nitrogen atmosphere, add toluene, triisobutylaluminum, and norbornene (m=0, R 1 ~R 4 Both are hydrogen) and polar comonomers (n is 9, FG is hydroxyl) with formula (IV) structure, stirring 8min, wherein, triisobutylaluminum and polar comonomers with formula (IV) structure The molar ratio is 20:1; After stirring, add triphenylcarbon tetrakis (pentafluorophenyl) borate, then add fluorenylamine titanium complex (R 5 is adamantyl, R 7 and R 8 is methyl, R 9 and R 14 is tert-butyl, R 6 , R 10 , R 11 , R 12 , R 13 , R 15 Both are hydrogen, X 1 and x 2 is methyl) for polymerization, the polymerization temperature is 25°C, and the polymerization time is 1min; wherein the molar ratio of the fluorenylamine titanium complex to the monomer is 1:1500, and the fluorenylamine titanium complex and triphenylcarbon tetra The molar ratio of (pentafluoropheny...
Embodiment 8-11
[0058] A kind of preparation method of functional cyclic olefin copolymer, concrete preparation steps are:
[0059] 1) Under a nitrogen atmosphere, add toluene, triisobutylaluminum, and norbornene (m=0, R 1 ~R 4 Both are hydrogen) and polar comonomers (n is 9, FG is hydroxyl) with formula (IV) structure, stirring 8min, wherein, triisobutylaluminum and polar comonomers with formula (IV) structure The molar ratio is 1.5:1; After stirring, add modified methyl aluminoxane, then add fluorenylamine titanium complex (R 5 is adamantyl, R 7 and R 8 is methyl, R 6 , R 9 , R 10 , R 11 , R 12 , R 13 , R 14 , R 15 Both are hydrogen, X 1 and x 2 is methyl) for polymerization, the polymerization temperature is 25°C, and the polymerization time is 1min; wherein the molar ratio of the fluorenylamine titanium complex to the monomer is 1:1500, the fluorenylamine titanium complex and the modified methyl The molar ratio of aluminoxane is 1:800;
[0060] 2) Pour the obtained reaction...
Embodiment 12-14
[0065] A kind of preparation method of functional cyclic olefin copolymer, concrete preparation steps are:
[0066] 1) Under a nitrogen atmosphere, add dichloroethane, trialkylaluminum, norbornene derivatives having a structure of formula (III) (m=1, R 1 is cyclopropanyl, R 2 is bromine, R 3 , R 4 is hydrogen) and the polar comonomer (n is 4, FG is carboxyl) with formula (IV) structure, stir 10min, wherein, the mole of trialkylaluminum and the polar comonomer with formula (IV) structure The ratio is 2.0:1, and the molar ratio of the norbornene derivative with the structure of formula (III) to the polar comonomer with the structure of formula (IV) is 1:1; after stirring, add modified methylalumoxane, Then add the fluorenylamine titanium complex (R 5 is tert-butyl, R 6 and R 7 is methyl, R 10 and R 13 is tert-butyl, R 8 , R 9 , R 11 , R 12 , R 14 , R 15 Both are hydrogen, X 1 and x 2 is methyl) to carry out polymerization reaction, the temperature of polymerizati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com