Low-temperature denitrification catalyst based on carbonized MOFs (metal organic frameworks) and preparation method thereof
A low-temperature denitration and catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc. Point reduction and other problems, to achieve the effect of easy operation, increased specific surface area, and favorable diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
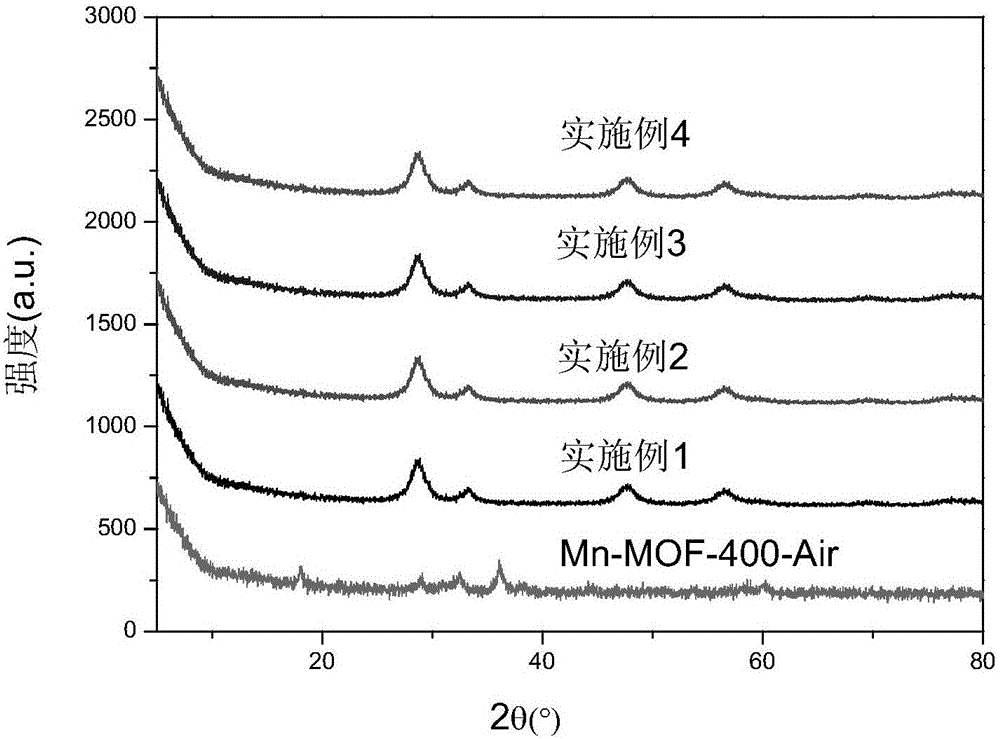
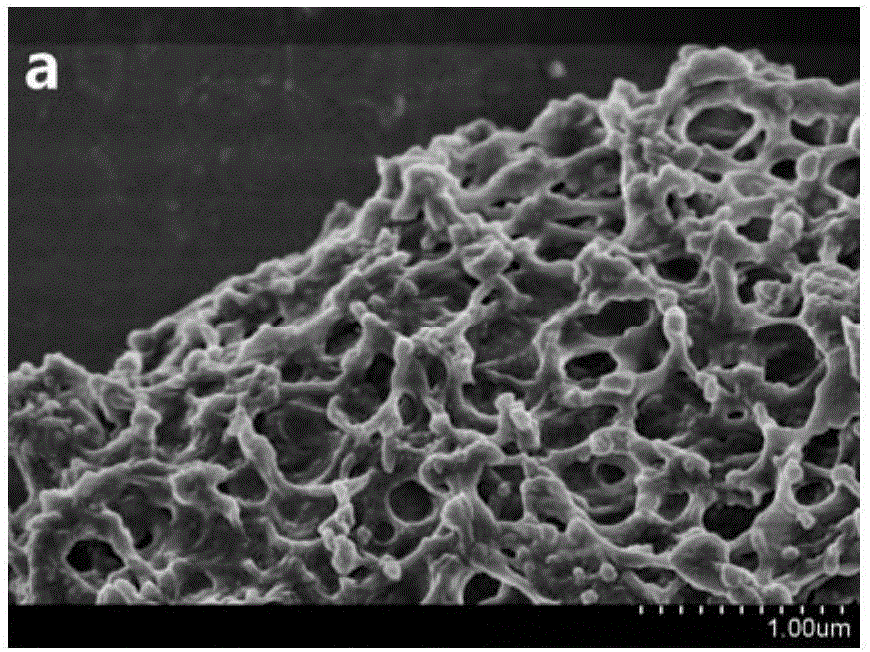
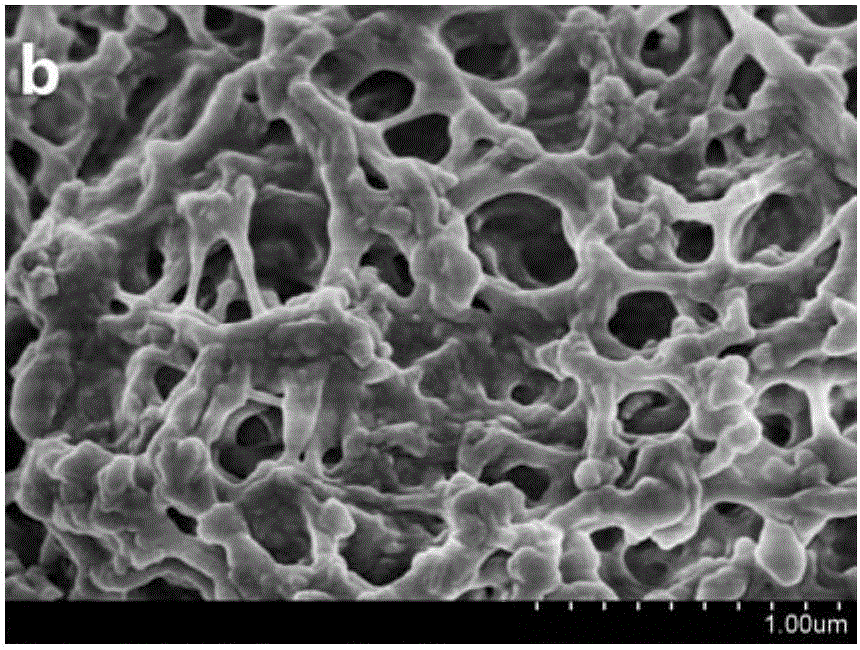
Embodiment 1
[0049] 520 μL of 50% Mn(NO 3 ) 2 solution and 1.94gCe(NO 3 ) 3 ·6H 2 O was added to DMF, 0.8mL of formic acid was added, and the solution was oscillated for 30min by ultrasonic wave until the solution was evenly mixed; then, the solvothermal synthesis reaction was carried out at 80°C for 24h to obtain the Mn-Ce bimetallic organic framework crystal material; the crystal material was washed with DMF and diethyl ether Finally, centrifugal filtration at 5000r / min was used for 5 minutes, and the product was dried in an oven at 100°C; the dried sample was carbonized in a muffle furnace with programmable temperature rise, and the temperature rise process was as follows: (1) Heating stage: first Raise the temperature from room temperature to 400°C with a heating rate of 1°C / min; (2) constant temperature stage: then keep at 400°C for 2 hours; (3) cooling stage: finally let the muffle furnace temperature drop from 400°C to 35.0°C , the cooling rate was 10°C / min, and a low-temperatur...
Embodiment 2
[0051] 520 μL of 50% Mn(NO 3 ) 2 solution and 3.88gCe(NO 3 ) 3 ·6H 2 O was added to DMF, 0.9mL formic acid was added, ultrasonically oscillated for 30min until the solution was mixed uniformly; then solvothermal synthesis reaction was carried out at 90°C for 20h to obtain the Mn-Ce bimetallic organic framework crystal material; the crystal material was subjected to DMF and diethyl ether in sequence Washing, using 5500r / min centrifugal filtration for 7min, put the product into a 100°C oven for drying; put the dried sample into a programmable temperature-raising muffle furnace for carbonization, and the temperature-programming process is as follows: (1) Heating stage: first Raise the temperature from room temperature to 400°C with a heating rate of 1°C / min; (2) constant temperature stage: then keep at 400°C for 2 hours; (3) cooling stage: finally let the muffle furnace temperature drop from 400°C to 35.0°C , the cooling rate was 10°C / min, and a low-temperature denitration ca...
Embodiment 3
[0053] 520 μL of 50% Mn(NO 3 ) 2 solution and 5.82gCe(NO 3 ) 3 ·6H 2 O was added to DMF, 1.0 mL of formic acid was added, ultrasonically oscillated for 30 min until the solution was evenly mixed; then solvothermal synthesis was carried out at 100°C for 16 h to obtain a Mn-Ce bimetallic organic framework crystal material; the crystal material was subjected to DMF and diethyl ether in sequence Washing, centrifugal filtration at 6000r / min for 8min, the product was dried in an oven at 100°C; the dried sample was carbonized in a muffle furnace with programmable temperature rise, and the temperature rise process was as follows: (1) Heating stage: first Raise the temperature from room temperature to 400°C with a heating rate of 1°C / min; (2) constant temperature stage: then keep at 400°C for 2 hours; (3) cooling stage: finally let the muffle furnace temperature drop from 400°C to 35.0°C , the cooling rate was 10°C / min, and a low-temperature denitration catalyst based on carbonized...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com