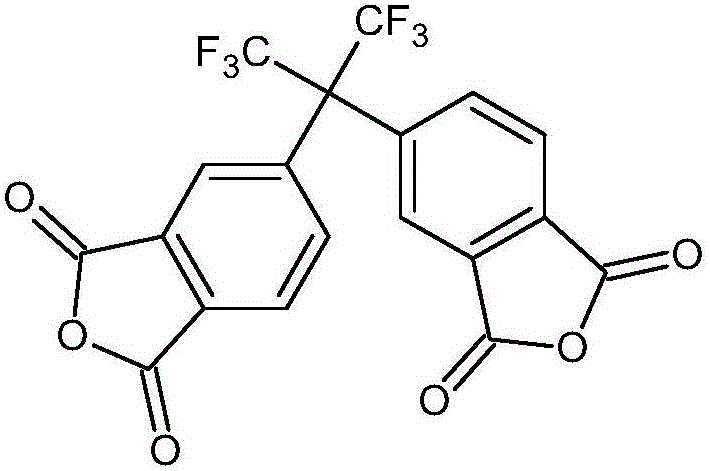
Synthetic method of 4,4'-(hexafluoroisopropylidene)diphthalic anhydride
A technology of diphthalic anhydride and hexafluoroisopropylidene, which is applied in the field of synthesis of 4,4'-diphthalic anhydride, can solve the problems of cumbersome operation, high amount of oxidant, and poor dehydration effect. Achieve the effects of lowering the reaction temperature, simplifying the operation steps and improving the efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example 1
[0027] In a 2000mL three-necked flask, add 14.40g of 4,4'-(hexafluoroisopropylidene)di-o-xylene and pyridine (200mL) / water (100mL) mixed solvent, heat to 100°C, then add permanganese Potassium permanganate 38.0g was reacted for 3 hours. After the reaction was finished, a small amount of ethanol was added dropwise to remove unreacted potassium permanganate. After the mixture was filtered, the filtrate was evaporated to remove pyridine, then the pH was adjusted to 1, the water was evaporated to dryness, 320 mL of acetone was added to heat to dissolve the organic matter, the insoluble matter was filtered off, and the filtrate was evaporated to dryness to obtain the crude product hexafluorotetraacid with a yield of 80.0%.
[0028] A mixed solvent of 4.80 g of hexafluorotetraacid, 8 mL of acetic anhydride, and 8 mL of xylene was added to a 150 mL flask, and reacted at 140° C. for 40 minutes. After the reaction, it was naturally cooled, and the crude product hexafluorodianhydride (6...
specific example 2
[0030] In a 2000mL three-necked flask, add 14.40g of 4,4'-(hexafluoroisopropylidene)di-o-xylene and pyridine (200mL) / water (100mL) mixed solvent, heat to 100°C, then add permanganese Potassium permanganate 50.0g was reacted for 4 hours. After the reaction was finished, a small amount of ethanol was added dropwise to remove unreacted potassium permanganate. After the mixture was filtered, the filtrate was evaporated to remove pyridine, then the pH was adjusted to 1, the water was evaporated to dryness, 320 mL of acetone was added to heat to dissolve the organic matter, the insoluble matter was filtered off, and the filtrate was evaporated to dryness to obtain the crude product hexafluorotetraacid with a yield of 62.0%.
[0031] A mixed solvent of 4.80 g of hexafluorotetraacid, 12 mL of acetic anhydride, and 12 mL of xylene was added to a 150 mL flask, and reacted at 110° C. for 40 minutes. After the reaction, it was naturally cooled, filtered to obtain the crude product hexaflu...
specific example 3
[0033] In a 2000mL three-necked flask, add 14.40g of 4,4'-(hexafluoroisopropylidene)di-o-xylene and pyridine (200mL) / water (100mL) mixed solvent, heat to 130°C, then add permanganese Potassium permanganate 50.0g was reacted for 2 hours. After the reaction was finished, a small amount of ethanol was added dropwise to remove unreacted potassium permanganate. After the mixture was filtered, the filtrate was evaporated to remove pyridine, then the pH was adjusted to 1, the water was evaporated to dryness, 320 mL of acetone was added to heat to dissolve the organic matter, the insoluble matter was filtered off, and the filtrate was evaporated to dryness to obtain the crude product hexafluorotetraacid with a yield of 40.0%.
[0034] A mixed solvent of 4.80 g of hexafluorotetraacid, 12 mL of acetic anhydride, and 12 mL of xylene was added to a 150 mL flask, and reacted at 130° C. for 1 hour. After the reaction, it was naturally cooled, and the crude product hexafluorodianhydride (6FD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com