Ochratoxin detoxification protein, encoding gene thereof and application
A technology of ochratoxin and gene, applied in the field of ochratoxin detoxification protein and its coding gene, can solve the problems of slow development of detoxification substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 Extraction of B.Subtilis genome
[0100] The extraction steps of B.Subtilis genome are as follows:
[0101] 1) Pick the activated B.Subtilis CW14 from LB solid medium, put it in 10mL LB liquid medium, and culture it at 37°C and 200r / min for 24h;
[0102] 2) Take 1mL of bacterial liquid in a 2mL centrifuge tube, centrifuge at 10000r / min for 5min, discard the supernatant; add 1mL of PBS buffer, resuspend the bacteria, centrifuge again, discard the supernatant;
[0103] 3) Add 500 μL of pre-cooled (stored at 4°C) ethanol, resuspend the bacteria, centrifuge at 10,000 r / min for 5 min, and discard the supernatant;
[0104] 4) Add 200 μL of PBS solution, 40 μL of 50 mg / mL lysozyme solution, pipette with a pipette gun, thoroughly suspend and precipitate, and bathe in 37°C water for 30 minutes;
[0105] 5) Add 500 μL lysate solution, 10 μL proteinase K solution, and bathe overnight at 55°C;
[0106] 6) Add 2 μL of RNase A solution and bathe in water at 37°C for 30 m...
Embodiment 2
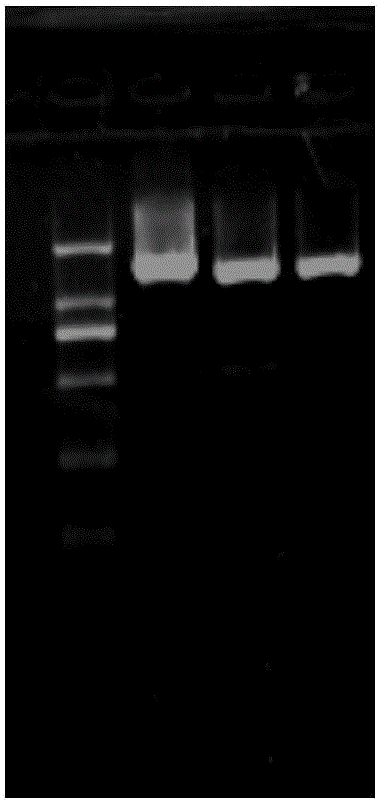
[0114] The PCR amplification of the CPA gene sequence of embodiment 2 B.Subtilis
[0115] (1) Primer synthesis
[0116] References (Chang XJ, Wu ZD, Wu SL. Degradation of ochratoxin A by Bacillusamyloliquefaciens ASAG1. Food Additives & Contaminants: Part A, 2015, 32(4):564–571), the primer sequences were slightly changed. The primer sequences are as follows:
[0117] F: 5'-CGC GTT GAA CATCAC GAA ATG GAA-3'
[0118] R: 5'-CCC AAA CCA GCC TGT TAC CG-3'
[0119] The above primers were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0120] (2) PCR amplification
[0121] Using a 25 μL reaction system, using B. Subtilis genomic DNA as a template, add in sequence to a 0.5 mL Eppendorf tube:
[0122]
[0123] After mixing and centrifuging for a short time, the amplification reaction was carried out according to the following PCR conditions: pre-denaturation at 95°C for 5 minutes; denaturation at 95°C for 30 seconds; annealing at 60°C for 1 minute; extension at 72°...
Embodiment 3
[0127] Example 3 Construction and Identification of Recombinant Plasmid pET-28a(+) / CPA
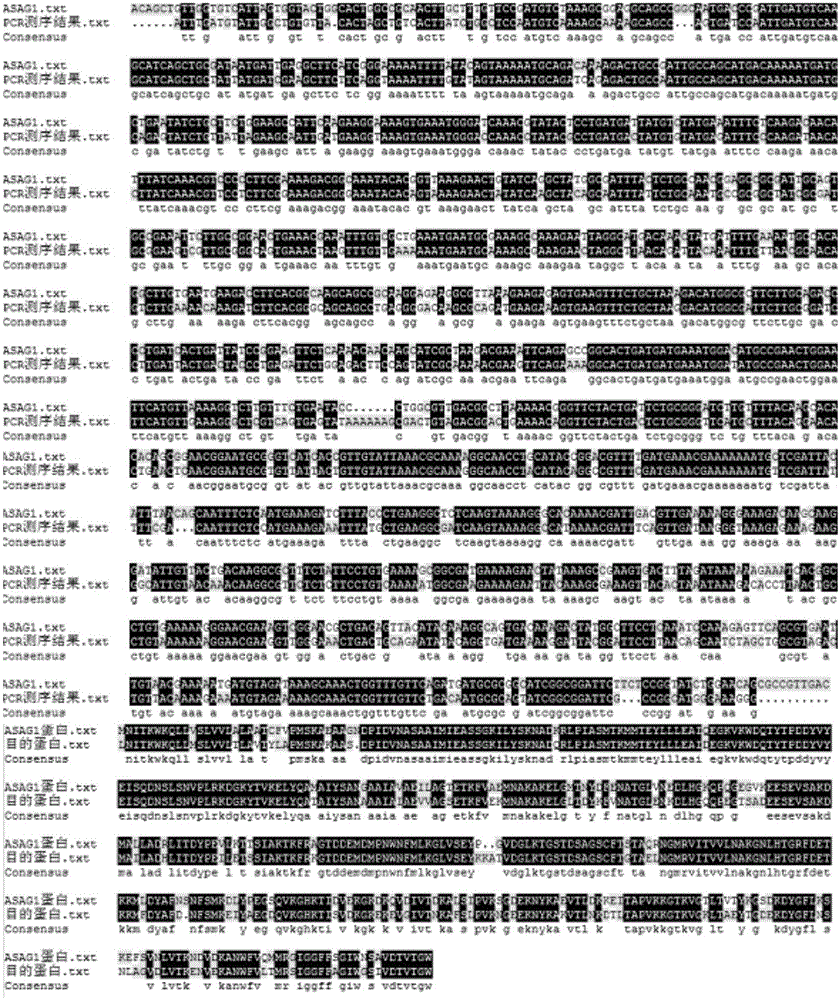
[0128] Codon optimization and construction of recombinant plasmid pET-28a(+) / CPA ( Figure 4 ) to Beijing Aoke Dingsheng Biotechnology Co., Ltd.
[0129] Using DNAMAN to compare the optimized synthetic target fragment with the carboxypeptidase sequence in B.Amyloliquefaciens ASAG1 in NCBI ( Figure 5 ), the results showed that the similarity of the optimized base sequence reached 77.02%, and the amino acid sequence remained unchanged.
[0130] 1. Activation of Glycerol bacteria containing recombinant plasmid pET-28a(+) / CPA
[0131] Thaw the Glycerolbacterium strain containing the recombinant plasmid pET-28a(+) / CPA frozen at -80°C on ice, and flick the wall tube to mix;
[0132] 2) Take 100 μL of the bacteria and inoculate into 2 mL of fresh LB / Kan (final concentration of Kan is 25 μg / mL) liquid medium, culture overnight on a shaker at 37°C at 200 r / min, and inoculate fresh LB / Kan (final...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com