Nonmetal oxygen reduction catalyst material as well as preparation method and application thereof
A non-metal and catalyst technology, which is applied in the field of non-metal oxygen reduction catalyst materials and its preparation, can solve the problems of nitrogen active site loss, function, difficult to activate nitrogen, etc., and achieve the promotion of catalysis, environmental friendliness and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
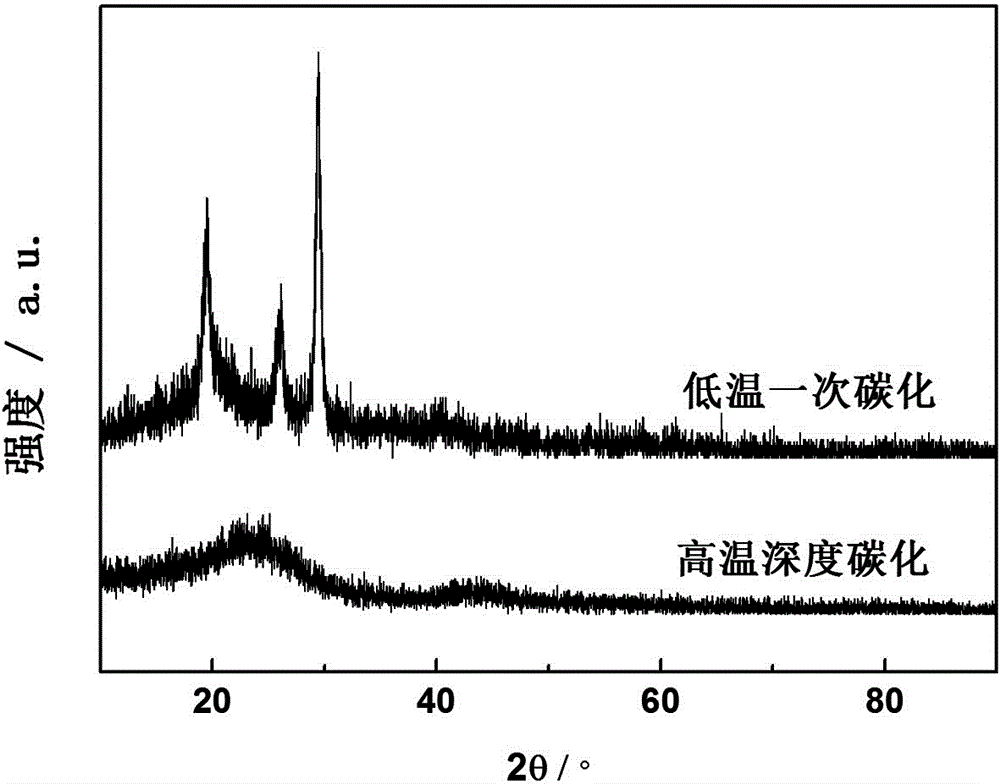
[0037] According to the mass, mix sucrose and melamine in a ratio of 2:1, add 4 g of sucrose and 2 g of melamine in a 400 ml beaker, mix and stir well, add 15 ml of concentrated sulfuric acid drop by drop, then put it into the autoclave, control the pressure Carbonization is 15Mpa at 200℃ for 6 hours. After the reaction is completed, it is filtered, washed, and dried at 75℃ to obtain the intermediate product; then the obtained intermediate product is subjected to a second carbonization reaction at 600℃ under the protection of nitrogen (high temperature deep carbonization) After 6 hours of treatment, a non-metal oxygen reduction catalyst material was obtained.
[0038] Physical characterization and performance testing of the intermediate product and non-metal oxygen reduction catalyst material prepared in this example are as follows:
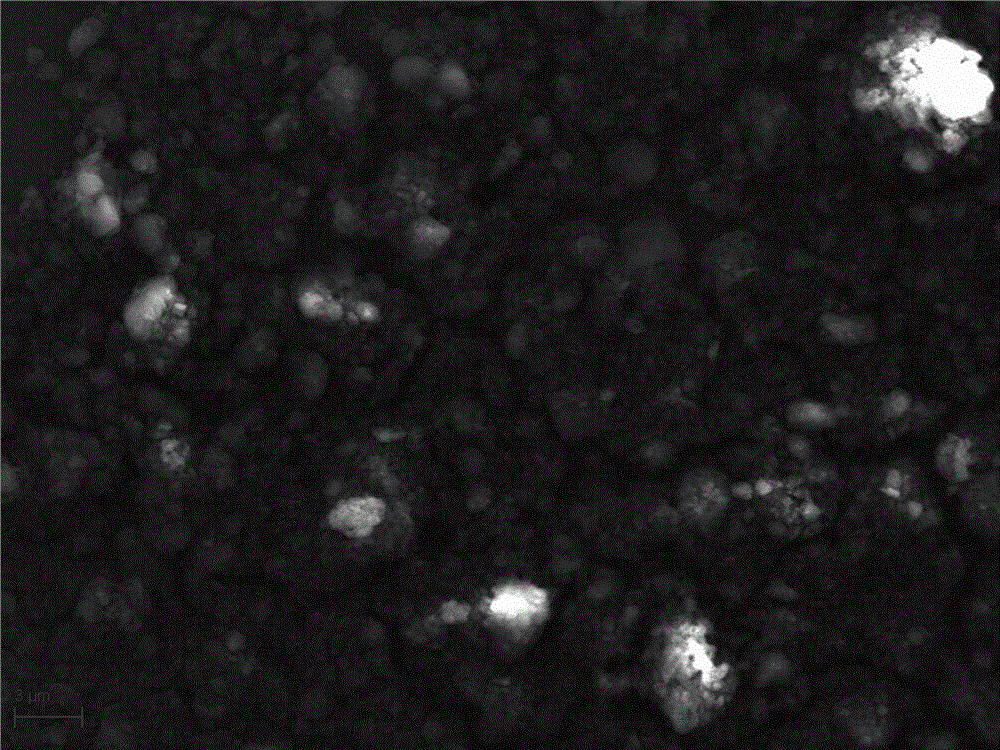
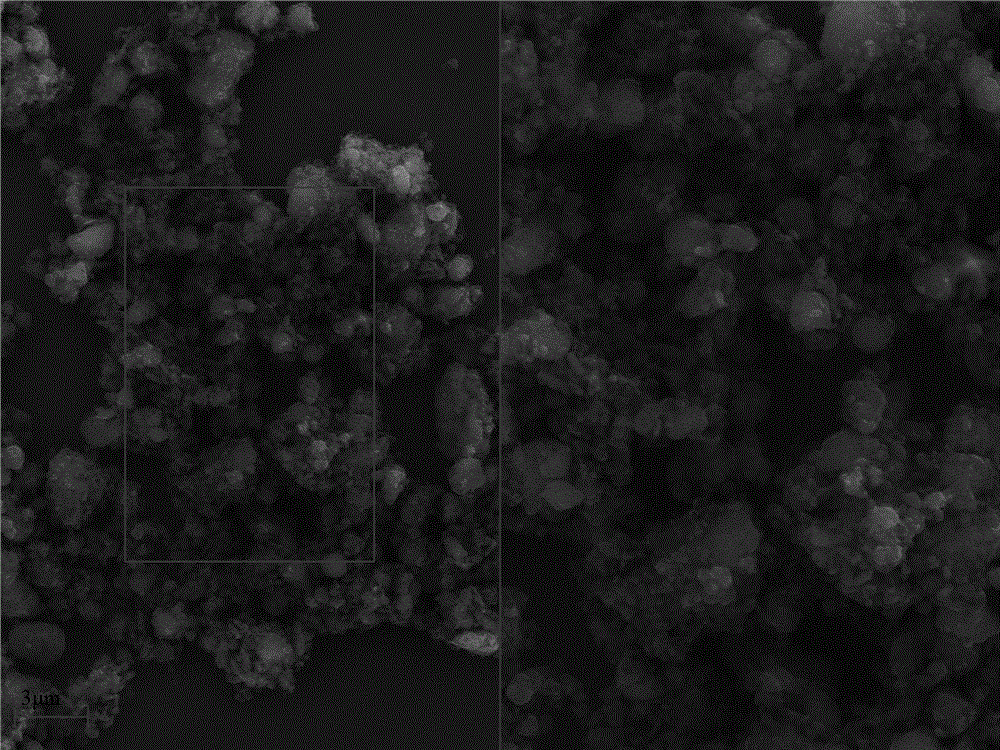
[0039] Such as figure 1 , figure 2 As shown, figure 1 It is a scanning electron microscope result image of the intermediate product obtained by the ...
Embodiment 2
[0044] According to the mass, mix sucrose and melamine in a ratio of 2:1, add 4 g of sucrose and 2 g of melamine in a 400 ml beaker, mix and stir evenly, add 10 ml of concentrated sulfuric acid drop by drop, then put it into the autoclave, control The pressure is 10 Mpa, 120 ℃ carbonization for 10 h once, after the reaction is completed, suction filtration, washing, and drying at 40 ℃, the intermediate product is obtained; then the obtained intermediate product is subjected to the second carbonization reaction at 600 ℃ under the protection of nitrogen (high temperature depth Carbonization) treatment for 2 h to obtain non-metallic oxygen reduction catalyst materials.
[0045] Similar to Embodiment 1, the non-metal oxygen reduction catalyst material prepared in this embodiment has similar performance advantages. Among them, the oxygen reduction test found that on the non-metal oxygen reduction catalyst material electrode prepared in this example, the initial potential and half-wave...
Embodiment 3
[0047] According to the mass, mix sucrose and melamine in a ratio of 2:1, add 4 g of sucrose and 2 g of melamine in a 400 ml beaker, mix and stir evenly, add 10 ml of concentrated sulfuric acid drop by drop, then put it into the autoclave, control The pressure is 10 Mpa, 140 ℃ carbonization for 10 h once, after the reaction is completed, suction filtration, washing, and drying at 50 ℃ to obtain the intermediate product; then the obtained intermediate product is under the protection of nitrogen, 800 ℃ secondary carbonization reaction (high temperature Deep carbonization) treatment for 2 h to obtain non-metallic oxygen reduction catalyst materials.
[0048] Similar to Embodiment 1, the non-metal oxygen reduction catalyst material prepared in this embodiment has similar performance advantages. Among them, the oxygen reduction test found that on the non-metal oxygen reduction catalyst material electrode prepared in this example, the initial potential and half-wave potential of the ox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com