A kind of gemini structure supramolecular gel factor and its preparation method
A supramolecular gel and gemini-type technology, applied in the direction of organic chemistry, can solve the problems of high cost, unfavorable wide application, and insufficient variety of gel materials, etc., and achieve low critical gel transition temperature and low critical gel concentration , Chiral induction promotes the regular arrangement of the system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
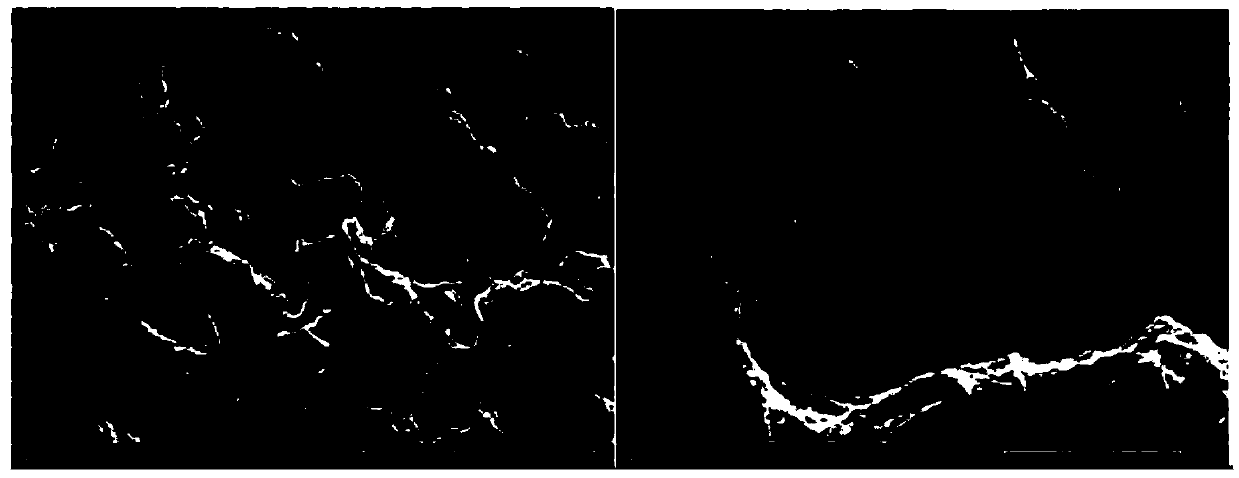
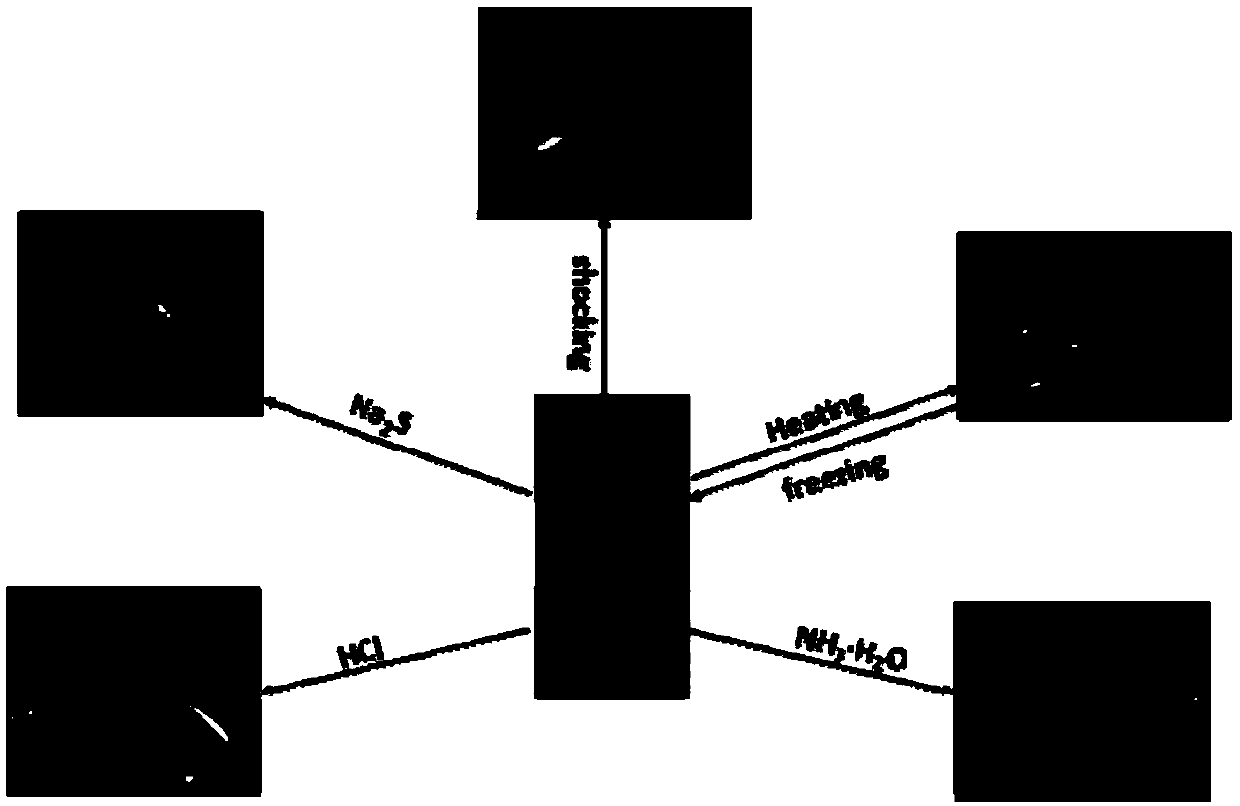
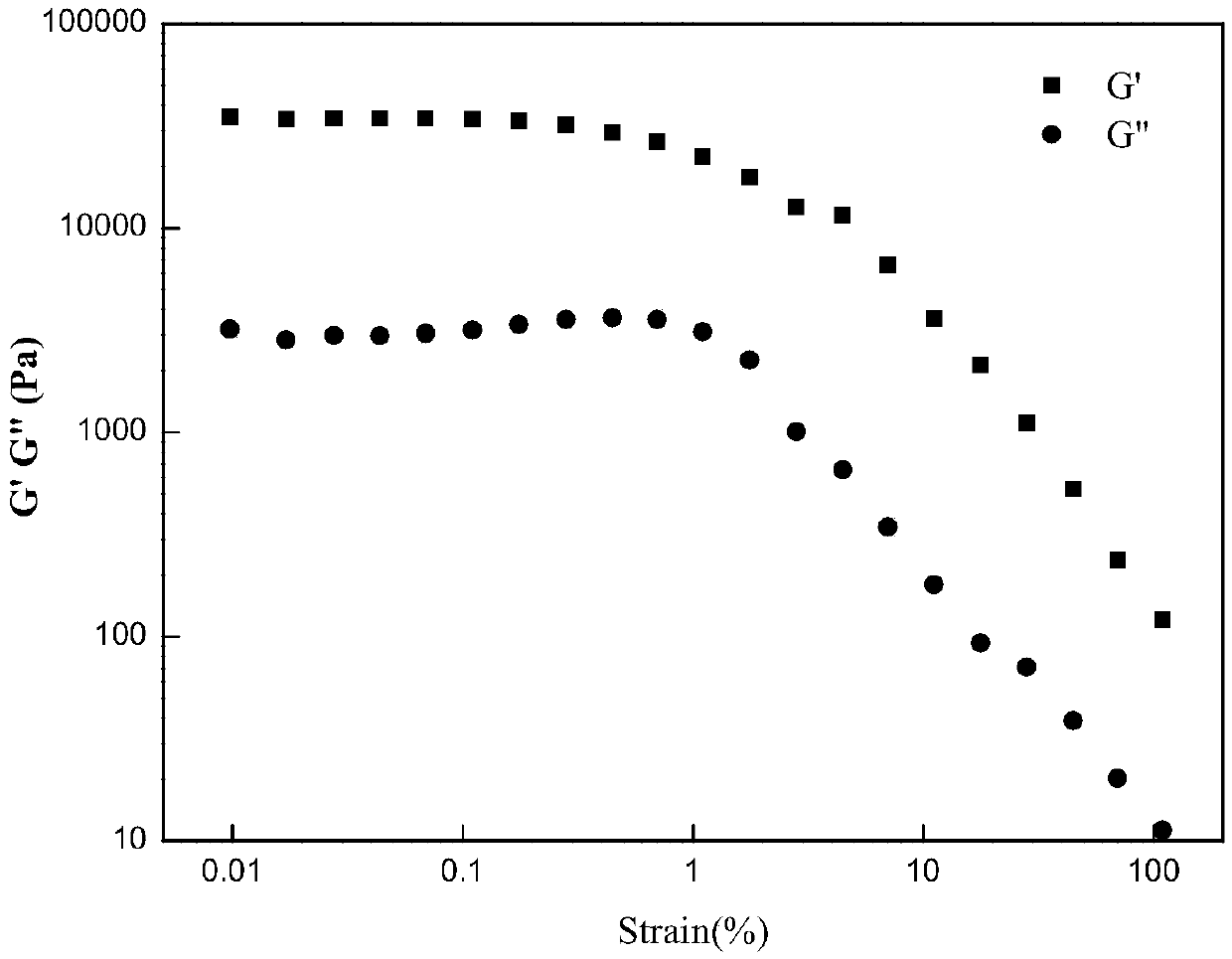
Image
Examples
Embodiment 1
[0032] Embodiment 1: (R 1 for m=8, R 3 for R 2 for )
[0033] 0.1mol L-alanine, 0.05mol Na 2 CO 3 Put the two raw materials into a three-necked flask, add 200.0mL of distilled water, stir at room temperature to dissolve, slowly add dropwise methanol solution containing 0.1mol pyridine-4-carbaldehyde, methanol 50mL, stir at room temperature for 5h, cool to -4°C, slowly add 0.4mmol NaBH 4 , stirred and reacted for 12 hours, adjusted the pH to 5-6, raised to room temperature and continued to stir for 3 hours, rotary evaporated to dryness, heated and dissolved with a large amount of absolute ethanol at 50°C and filtered, and the filtrate was rotary evaporated to obtain N-(4-pyridine methyl)-L-alanine.
[0034] FT-IR: (cm -1 ): 3375(NH), 3225(OH), 3019(CH), 1720(C=O), 1650(C=N).
[0035] 1 H-NMR (500MHz, DMSO): δ8.597~8.606 (d, J=4.5Hz, 2H, C 5 h 4 N), 8.529~8.541 (d, J=6Hz, 2H, C 5 h 4 N), 4.295~4.300 (d, J=2.5Hz, 2H, -CH 2 -),3.735~3.778(q,1H,CH),1.525~1.539(d,...
Embodiment 2
[0040] Embodiment 2: (R 1 for m=12,R 3 for R 2 for )
[0041] 0.1mol L-valine, 0.05mol Na 2 CO3 Put the two raw materials into a three-necked flask, add 200mL of distilled water, stir at room temperature to dissolve, slowly add dropwise methanol solution containing 0.1mol benzaldehyde, methanol 50mL, stir at room temperature for 5h, cool to -4°C, slowly add 0.4mmol NaBH 4 , stirred and reacted for 12 hours, adjusted the pH to 5-6, raised to room temperature and continued to stir for 2 hours, rotary evaporated to anhydrous, added ethanol, heated to dissolve and filtered, and the filtrate was rotary evaporated to obtain N-(benzyl)-L-valine.
[0042] FT-IR (cm -1 ):3575(NH), 3400(O-H), 1650(-C=N-), 1600(-C=C-).
[0043] 1 H-NMR(500MHz,DMSO):δ7.2746~7.3243(q,4H,C 6 h 4 ), 7.2011~7.2284 (d, J=13.6Hz1H, C 6 h 4 ),3.456~3.728(dd,2H,-CH 2 -), 2.768~2.780(d, J=6Hz, 1H, CH), 1.766~1.940(m, 1H, -CH(CH 3 ) 2 ), 0.910~0.933 (d, J=11.5Hz, 6H, -CH 3 ).
[0044] Dissolve ...
Embodiment 3
[0048] Embodiment 3: (R 1 for m=12,R 3 for R 2 for )
[0049] 0.1mol L-valine, 0.05mol Na 2 CO 3 Put the two raw materials into a three-necked flask, add 200mL of distilled water, stir at room temperature to dissolve, slowly add dropwise methanol solution containing 0.1mol pyridine-4-carbaldehyde, 50mL of methanol, stir at room temperature for 5h, cool to -4°C, and slowly add 0.4mol NaBH 4 , stirred and reacted for 12 hours, adjusted the pH to 5-6, raised to room temperature and continued to stir for 5 hours, rotary evaporated to anhydrous, added ethanol, heated to dissolve and filtered, and the filtrate was rotary evaporated to obtain N-(4-pyridylmethyl)-L-valine .
[0050] FT-IR (cm -1 ;): 3385(NH), 3255(OH), 3069(CH), 2987(CH), 1690(C=O), 1667(C=C), 1430(C=N).
[0051] 1 H-NMR (500MHz, DMSO): δ8.492~8.504 (d, J=6Hz, 2H, C 5 h 4 N), 7.362~7.372 (d, J=5Hz, 2H, C 5 h 4 N),3.582~3.903(dd,2H,-CH 2 -),2.828~2.839(d,J=5.5,1H,-CH),1.879~1.944(m,1H,-CH(CH 3 ) 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com