An injectable nonionic superhydrogel based on oligomeric amino acid amphiphiles
A technology of amino acid and oligoethylene glycol, applied in the field of materials, can solve the problems of unfavorable large-scale production, high cost, cumbersome and complicated process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1, the preparation of polyamino acid-based amphiphile molecule and its hydrogel shown in formula V belonging to formula I
[0089]
[0090] (1) Get 10 grams of γ-benzyl-L-glutamate and 5 grams of triphosgene into the reaction flask, dissolve the two with 200 milliliters of tetrahydrofuran under nitrogen protection conditions, heat up to 50 ° C for 4 hours, The system changed from cloudy to clear, and the solvent was drained to obtain a light yellow solid, which was recrystallized three times with tetrahydrofuran and n-hexane to obtain 8.93 g of white needle-like crystals, with a yield of 80.47%.
[0091] (2) The ring-opening polymerization reaction of this step is carried out in a reaction flask under nitrogen protection: take 3 grams of the white product of step (1), dissolve it in 60 milliliters of tetrahydrofuran, and prepare a 50 mg / ml solution. Quickly add 0.42 g of dodecylamine. In this system, the mole fraction ratio of the white product obtained i...
Embodiment 2
[0100]Example 2, the preparation of polyamino acid-based amphiphile molecule and its hydrogel represented by formula VI belonging to formula II
[0101]
[0102] (1) Get 10 grams of γ-benzyl-L-glutamate and 5 grams of triphosgene into the reaction flask, dissolve the two with 200 milliliters of tetrahydrofuran under nitrogen protection conditions, heat up to 50 ° C for 4 hours, The system changed from cloudy to clear, and the solvent was drained to obtain a light yellow solid, which was recrystallized three times with tetrahydrofuran and n-hexane to obtain 9.00 g of white needle-like crystals, with a yield of 81.10%.
[0103] (2) The polymerization reaction of this step is carried out in a reaction flask under nitrogen protection: take 2.37 grams of the white product of step (1), dissolve it in 45 milliliters of tetrahydrofuran, and add 2 milliliters of dimethylimide to help dissolve it, and prepare into a solution of approximately 50 mg / ml. Quickly add 0.55 g of dodecylam...
Embodiment 3
[0108] Example 3, the preparation of polyamino acid-based amphiphile molecules and their hydrogels represented by formula VII belonging to formula IV
[0109]
[0110] (1) Get 0.312 grams of hexadecyl-glutamic acid tripeptide compound and dissolve in 3 milliliters of dimethyl sulfoxide, then add 0.460 grams of EDC and 0.276 grams of NHS amidation reagent, and react at 25°C for 24 hours, The activated ester of cetyl-glutamate tripeptide is obtained.
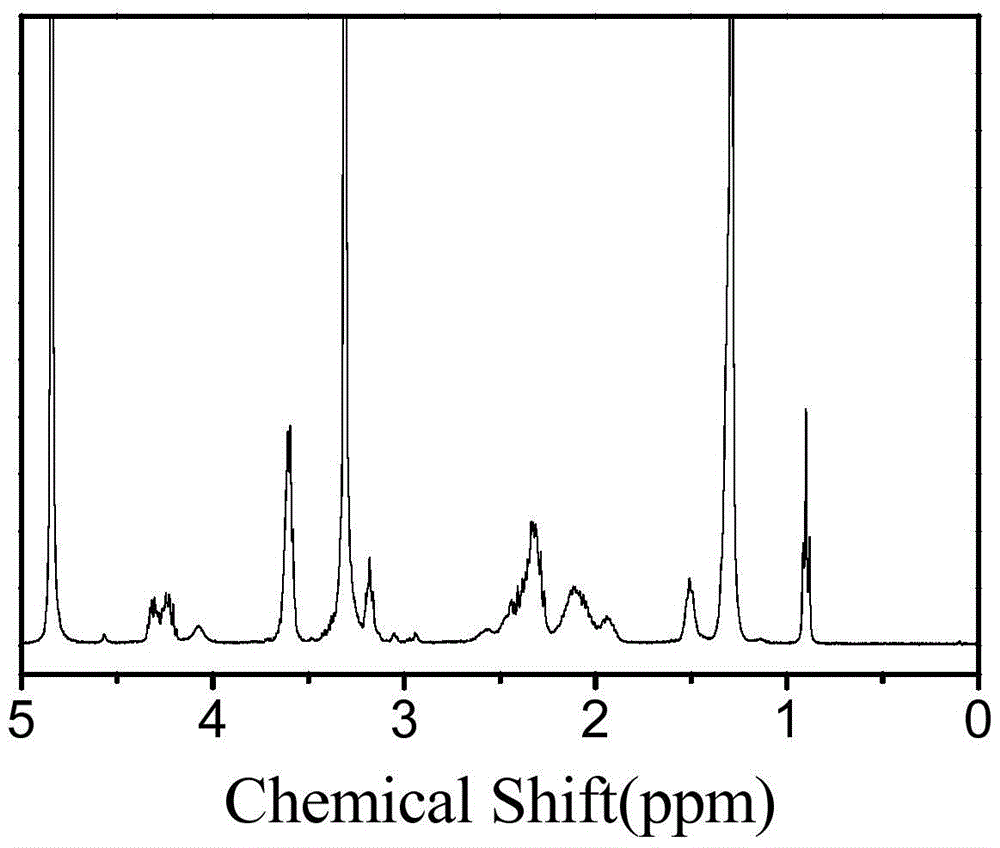
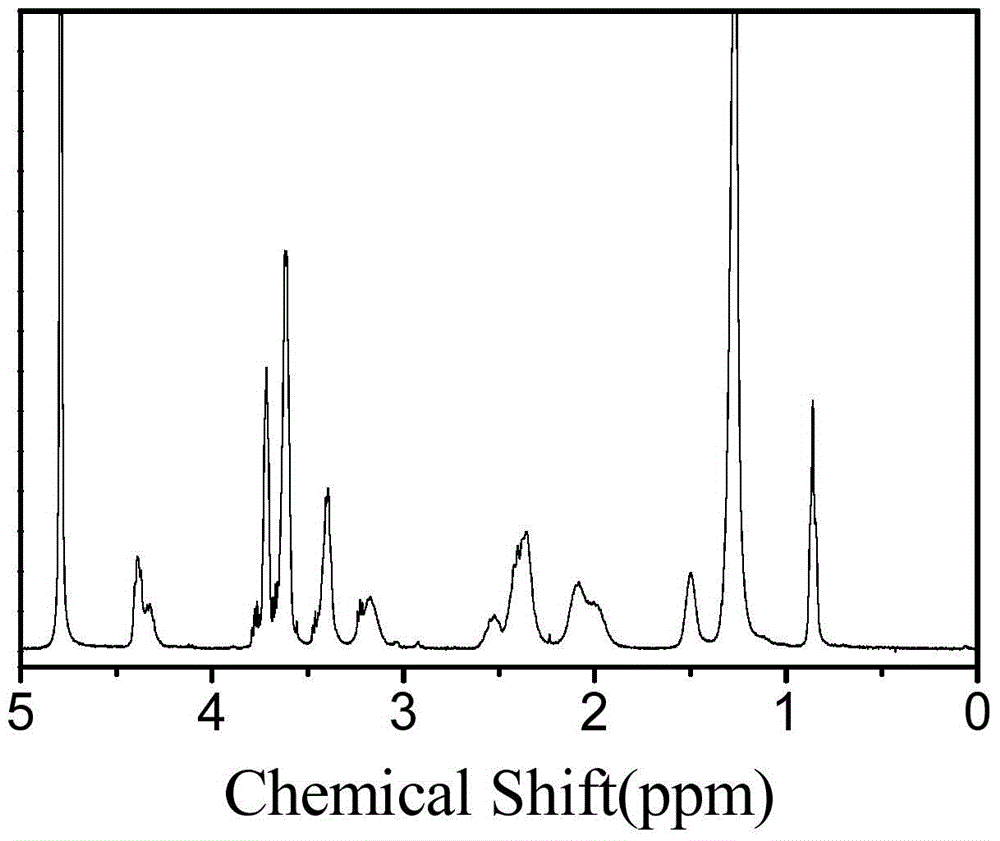
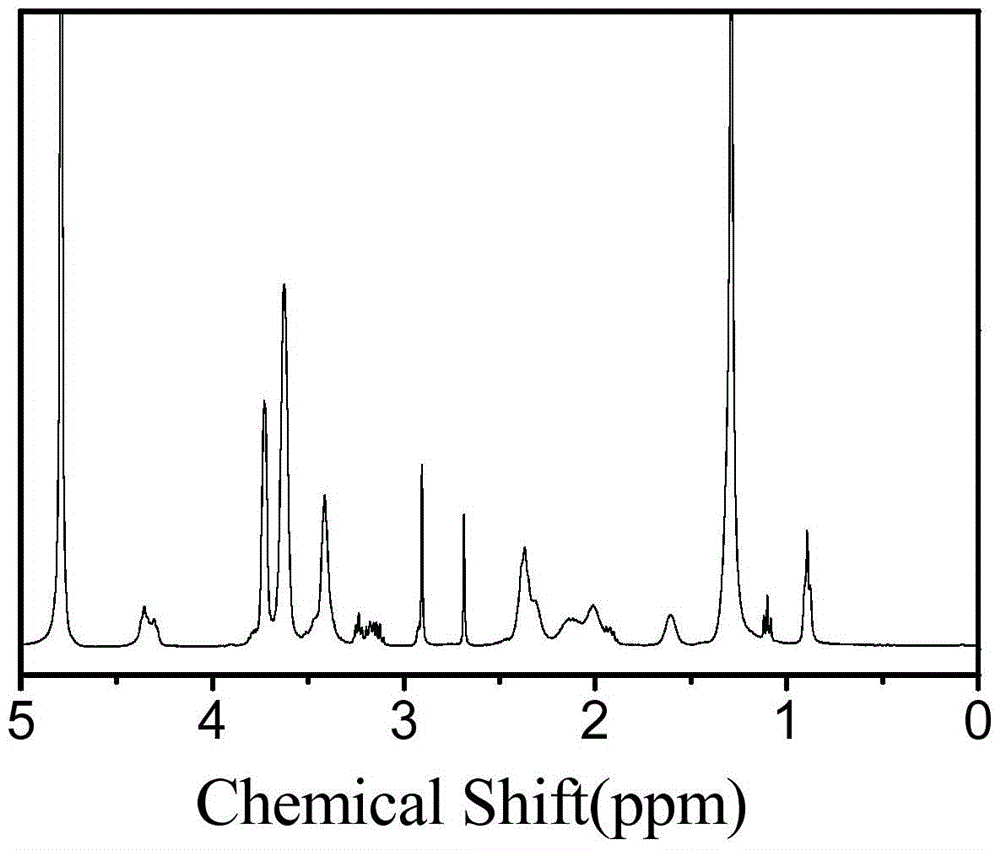
[0111] (2) Add 0.252 g of diethylene glycol amine to the reaction solution in step (1), continue the reaction at 25°C for 24 hours, remove the solvent in the system by rotary evaporation, re-dissolve the product with methanol, precipitate a large amount of ether, and centrifuge A white solid was obtained and dried to obtain a white solid powder product. of the product 1 H-NMR spectrum as image 3 shown. It can be seen from the figure that the product has a correct structure and is the target compound.
[0112] (3) Dissolve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com