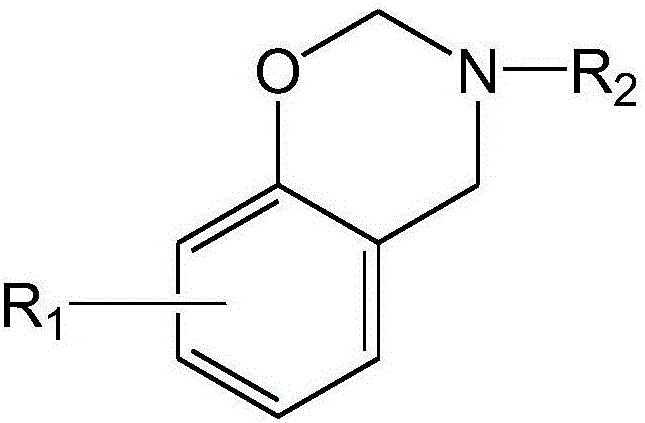
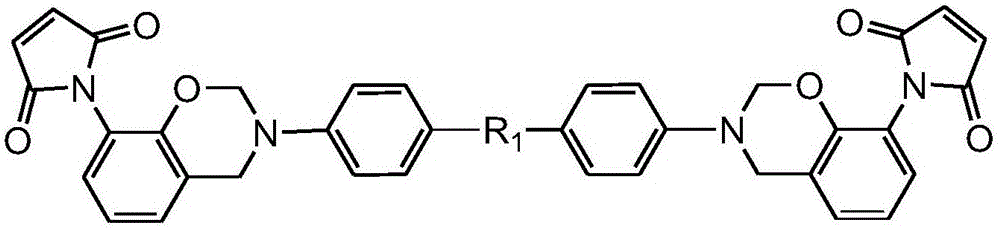
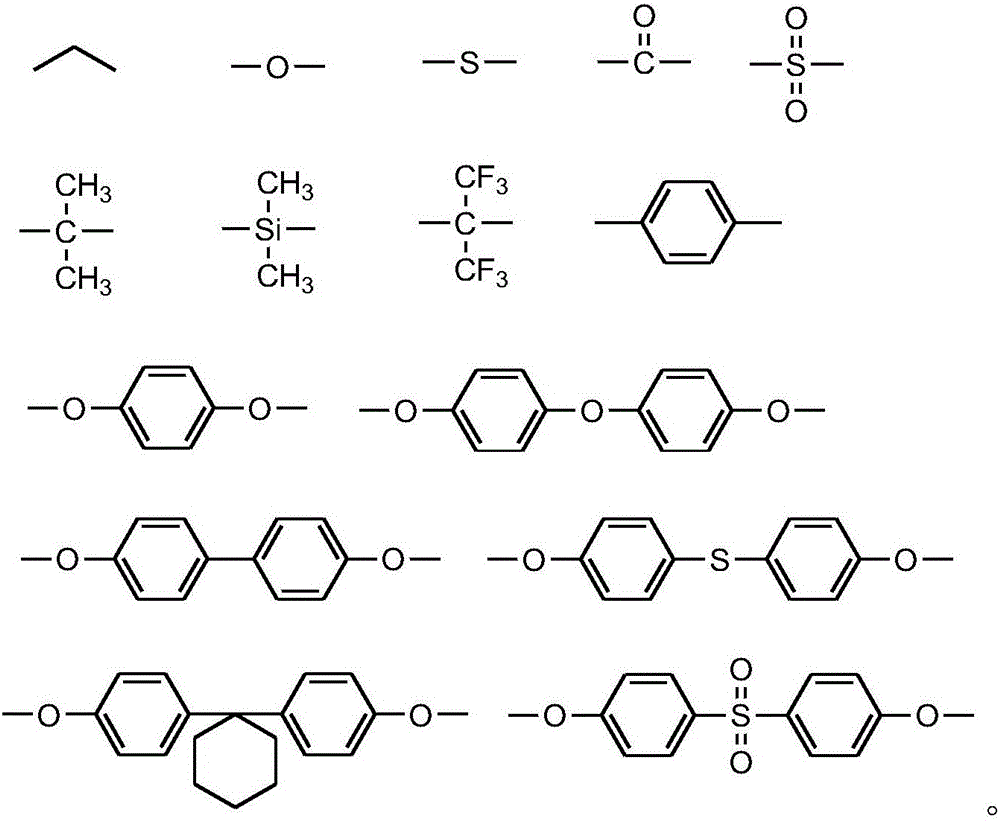
Dibenzoxazine monomer containing ortho-position maleimide groups and preparation method thereof
A technology of maleimide group and maleimide function, which is applied in the field of bisbenzoxazine monomer and its preparation, can solve the problem of low yield, long reaction time, and no reports involving ortho horses Solve problems such as the synthesis and use of imide bis-benzoxazine, and achieve the effects of excellent overall performance of the resin, reduced synthesis reaction time, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 30.6g of 2-aminophenol and 30g of maleic anhydride were added to the ice bath system of 80mL dimethylformamide (DMF), then 15g of phosphorus pentoxide and 8g of concentrated sulfuric acid dissolved in 50mL of DMF were added thereto, and the reaction After 30 minutes, the reaction device was placed in an oil bath at 70° C. for 6 hours. After the reaction, the reaction solution was poured into 200ml of deionized water to obtain a large amount of precipitation. After suction filtration, it was washed three times with a large amount of water and ethanol, and dried in a vacuum oven at 50° C. to obtain 43.5 g of the product ortho-maleimide functionalized phenol with a yield of 82%.
[0030] Weigh 30g of ortho-maleimide phenol obtained in the previous step, 15.7g of 4,4'-diaminodiphenylmethane, 9.5g of paraformaldehyde, and 80ml of toluene, respectively, and add them to In the reaction bottle of the tube, heat at a rate of 10°C / h to 120°C for 6h. After the reaction was finis...
Embodiment 2
[0032] The ortho-maleimide functionalized phenol was prepared referring to the operation steps of Example 1.
[0033] Add 30g of o-maleimide phenol, 16.8g of 4,4'-diaminobenzophenone, 9.5g of paraformaldehyde, and 80ml of xylene into a reaction flask equipped with a stirrer, a thermometer, and a condenser , heated to 130°C at a rate of 10°C / h for 6h. After the reaction was finished, deionized water was added to the reaction solution for precipitation, and a large amount of precipitate was obtained. The precipitate was washed 3 times with 5% sodium hydroxide solution, then washed with water, filtered, and dried under vacuum for 12 hours to obtain Product 46.4g, yield 92%.
Embodiment 3
[0035] The ortho-maleimide functionalized phenol was prepared referring to the operation steps of Example 1.
[0036] Add 30g of o-maleimide phenol, 17.2g of 4,4'-diaminodisulfide, 9.5g of paraformaldehyde, and 80ml of xylene into a reaction flask equipped with a stirrer, a thermometer and a condenser, respectively. Heating to 120°C at a rate of 10°C / h for 6h. After the reaction was finished, deionized water was added to the reaction solution for precipitation, and a large amount of precipitate was obtained. The precipitate was washed 3 times with 5% sodium hydroxide solution, then washed with water, filtered, and dried under vacuum for 12 hours to obtain Product 41.1g, yield 81%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com