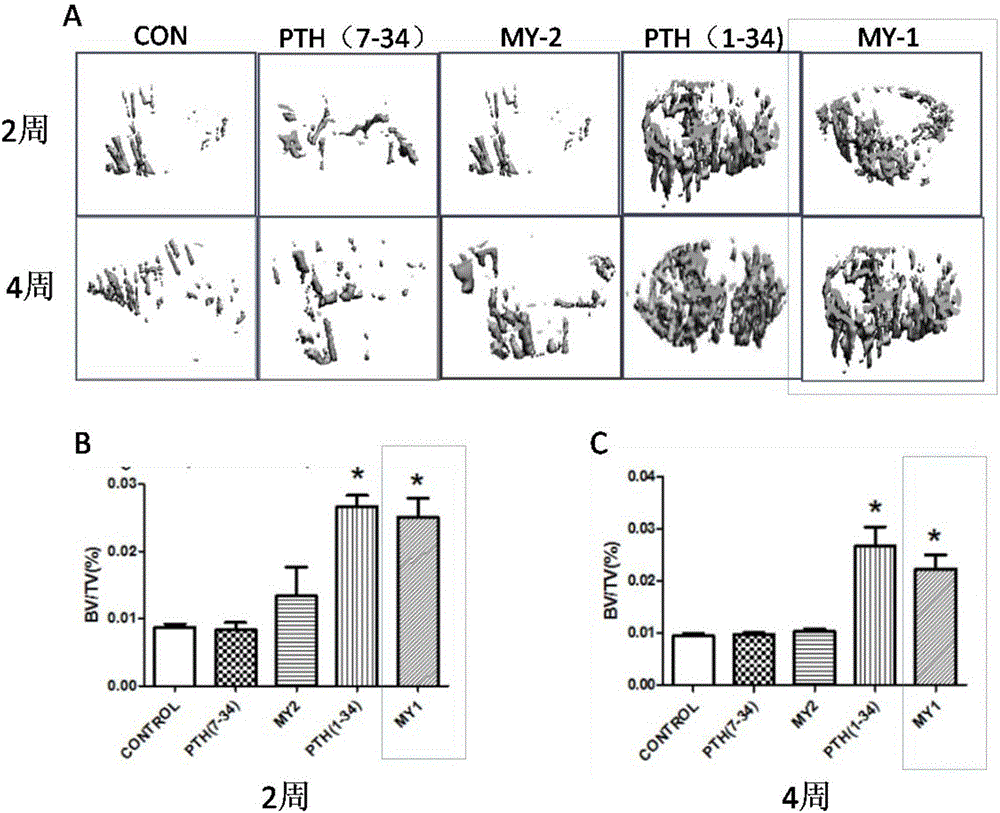
PTH mimic peptide and application thereof
A technology that mimics peptides and amino acids, applied in the biological field, can solve problems such as unachieved effects, and achieve the effects of promoting fracture healing, promoting osteoblast differentiation, and improving clinical medicinal value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
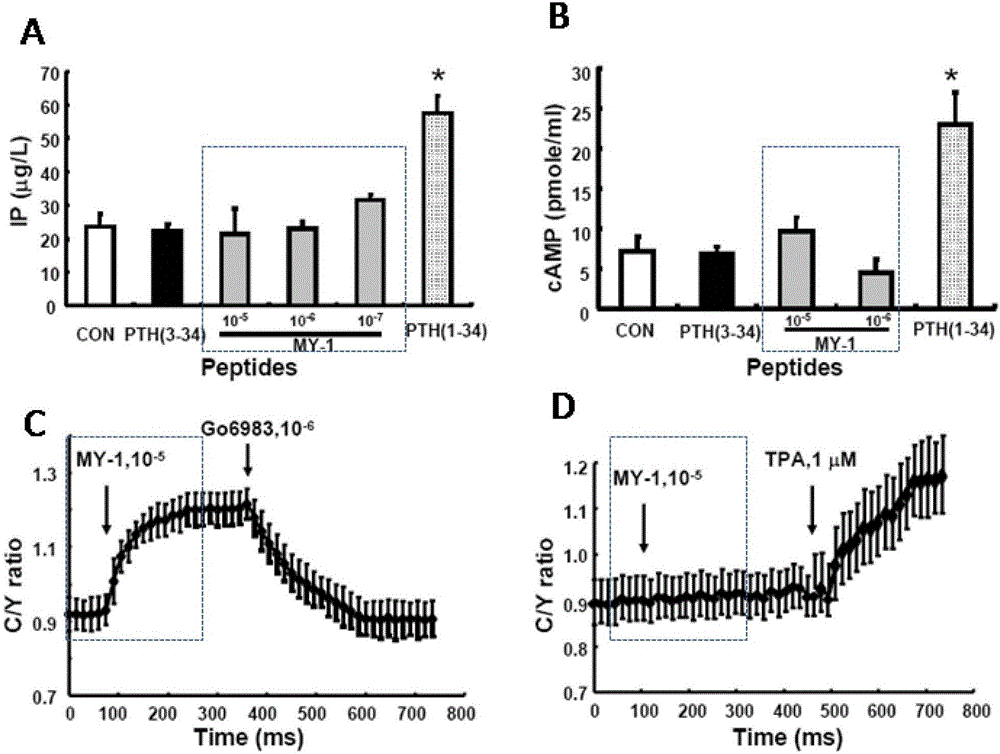
[0033] The PTH receptor (PTHR1) gene was transfected into HEK293 cell line (not expressing PTHR1), and cell clones stably expressing PTHR1 were selected (the PTHR1 receptor gene has been inserted into the genome of HEK293 cells, and the expression of the receptor gene is stable; hereinafter referred to as HEK293- PTHR1 cells). For experimental results, see figure 1 .
[0034] From figure 1 A can see that (10 -5 M) PTH (3-34), MY-1 peptide (10 -5 M, 10 -6 M) Stimulation for 4 hours could not activate the PLC signal in HEK293-PTHR1 cells to increase the production of IP, but PTH(1-34) had the function of activating PLC. figure 1 B shows PTH(3-34)(10 -5 M), MY-1 peptide (10 -5 M, 10 -6 M) 4-hour stimulation fails to promote cAMP production in HEK293-PTHR1 cells.
[0035] In order to detect the activation of PKC in cells, we transfected HEK193-PTHR1 cells (HEK193-PTHR1-CKAR cells) with PKC activation fluorescent detection molecule CKAR (Note: If PKC is activated, it will ...
Embodiment 2
[0040] Example 2: Inhibition of osteoblast apoptosis by MY-1 peptide and MMY-1
[0041] The osteoblast cell line MC3T3-E1 was treated with 4x10 5 Inoculate such as 24-well plate at the density of each well, and when the cell density in the well reaches 80% (leading to microscope observation and measurement), replace the 10% serum + α-MEM medium with 1% serum + α-MEM medium to continue culturing 24 hours, 10 -6 MY-1 peptide of M, 10 -6 MMY-1 peptide of M and 10 -8 M PTH (1-34) was added with 1% serum + α-MEM medium to continue culturing for 4 hours, then digested with 0.05% trypsin to prepare suspension cells, PI (stained damaged nuclei) and FITC (stained damaged nuclei) Cell membrane) staining solution was incubated at 4°C for 20 minutes, and the flow cytometer classified and counted the percentages of cells according to the staining conditions of PI and FITC, among which PI + FITC + The cell population is late apoptotic or dead cells, PI - FITC + early apoptotic cells....
Embodiment 3
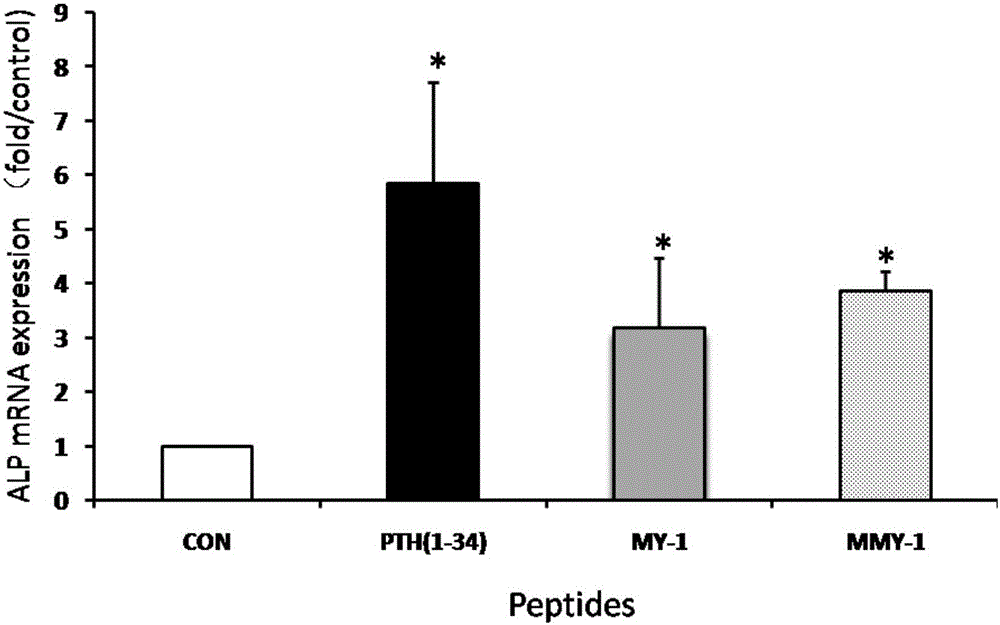
[0045] Example 3: MY-1 peptide and MMY-1 promote osteoblast expression of osteogenic gene alkaline phosphatase
[0046] Inoculate the osteoblast cell line MC3T3-E1 with a density of 1x106 / hole, such as a 6-well plate, and when the cell density in the well reaches 80% (leading to microscope observation and measurement), replace the 10% serum + α-MEM medium with 1% Serum+α-MEM medium continued to culture for 24 hours, 10 -6 MY-1 peptide of M, 10 -6 MMY-1 peptide of M and 10 -8 MPTH (1-34) was added to α-MEM medium to continue culturing for 4 hours, then total RNA was extracted, and the expression of alkaline phosphatase (ALP) was detected by quantitative PCR.
[0047] Such as figure 2 As shown, PTH(1-34), MY-1 peptide and MMY-1 peptide can significantly promote the expression of osteoblast osteogenesis gene ALP.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com