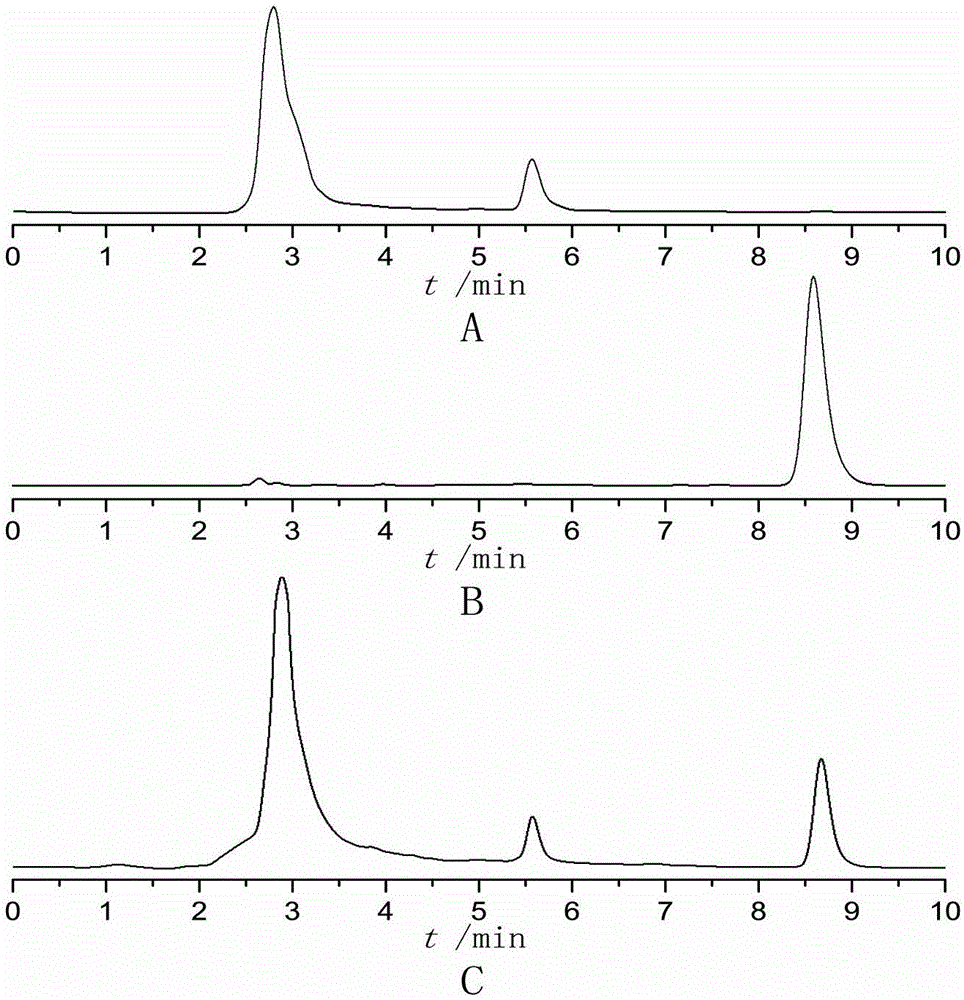
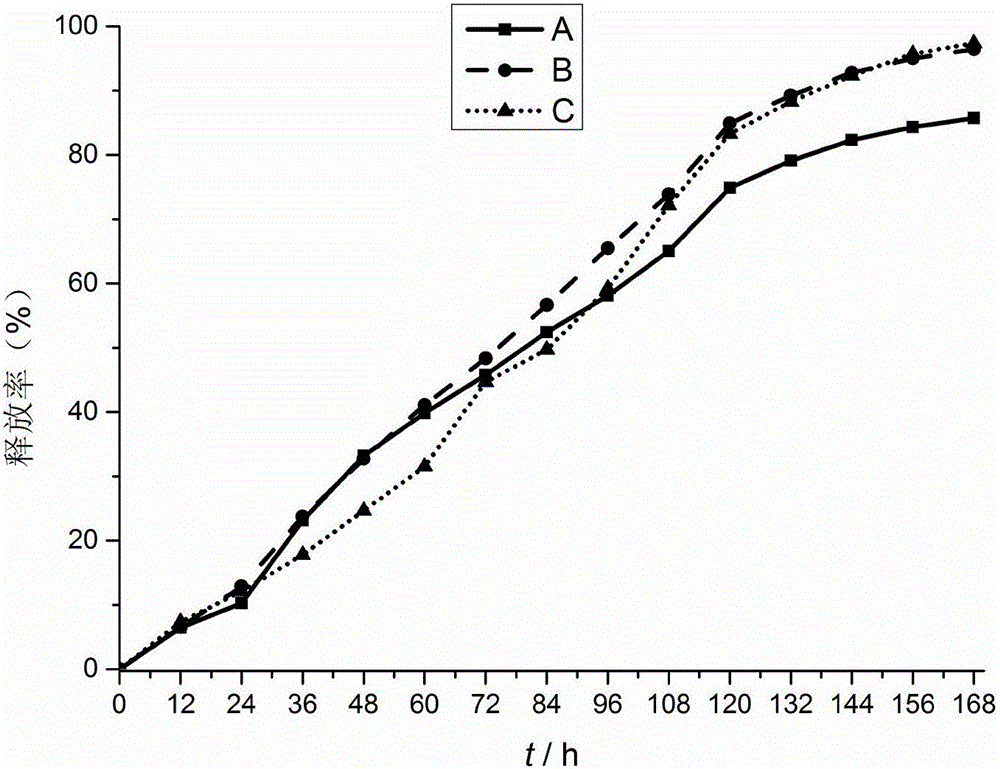
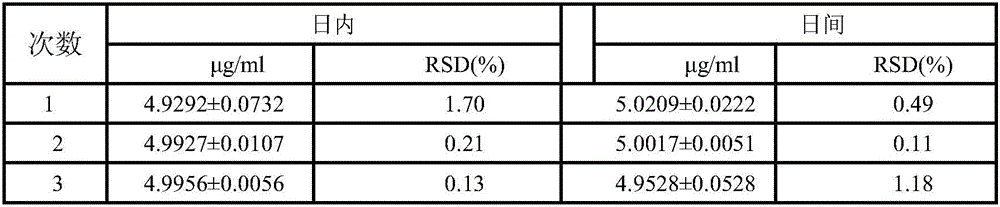
Biological protein glue-triamcinolone acetonide slow release agent as well as preparation method and application of slow release agent
A technology of biological protein glue and biological protein, which is applied in the field of bioprotein glue-triamcinolone acetonide sustained-release agent and its preparation, can solve the problems of puncture bleeding, increase patient pain and economic burden, and different curative effects, and achieve the goal of prolonging the dissolution time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of bioprotein glue-triamcinolone acetonide sustained-release agent
[0035] 1) Configure aprotinin solution
[0036] Take 100 mg of aprotinin, dissolve it with 900 μl of normal saline in a sterile, enzyme-free environment, and mix well to obtain an aprotinin solution;
[0037] 2) Configure the main body glue solution of biological protein
[0038] According to the instruction manual of the purchased medical biological protein glue, dissolve the main glue with the supporting main glue solution to obtain the biological protein main glue solution;
[0039] 3) Configure the catalyst solution
[0040] According to the instruction manual of the purchased medical biological protein glue, dissolve the catalyst (thrombin) with the supporting calcium chloride solution to obtain the catalyst (thrombin) solution;
[0041] 4) Take the matching first syringe, extract 1ml of the biological protein main glue solution prepared in step 2), 10 μl of the aprotinin...
Embodiment 2
[0042] Example 2: Preparation of bioprotein glue-triamcinolone acetonide sustained-release agent
[0043] 1) Configure aprotinin solution
[0044] Take 100 mg of aprotinin, dissolve it with 900 μl of normal saline in a sterile, enzyme-free environment, and mix well to obtain an aprotinin solution;
[0045]2) Configure the main body glue solution of biological protein
[0046] According to the instruction manual of the purchased medical biological protein glue, dissolve the main glue with the supporting main glue solution to obtain the biological protein main glue solution;
[0047] 3) Configure the catalyst solution
[0048] According to the instruction manual of the purchased medical biological protein glue, dissolve the catalyst (thrombin) with the supporting calcium chloride solution to obtain the catalyst (thrombin) solution;
[0049] 4) Take the matching first syringe, extract 1ml of the biological protein main glue solution prepared in step 2), 10 μl of the aprotinin ...
Embodiment 3
[0050] Example 3: Preparation of bioprotein glue-triamcinolone acetonide sustained-release agent
[0051] 1) Configure aprotinin solution
[0052] Take 100 mg of aprotinin, dissolve it with 900 μl of normal saline in a sterile, enzyme-free environment, and mix well to obtain an aprotinin solution;
[0053] 2) Configure the main body glue solution of biological protein
[0054] According to the instruction manual of the purchased medical biological protein glue, dissolve the main glue with the supporting main glue solution to obtain the biological protein main glue solution;
[0055] 3) Configure the catalyst solution
[0056] According to the instruction manual of the purchased medical biological protein glue, dissolve the catalyst (thrombin) with the supporting calcium chloride solution to obtain the catalyst (thrombin) solution;
[0057] 4) Take the matching first syringe, extract 1ml of the biological protein main glue solution prepared in step 2), 10 μl of the aprotinin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com