Preparation method of acyloin compounds
A compound and acetoin technology are applied in the field of preparation of acetoin compounds, can solve problems such as instability of catalyst thiazole salt, influence on acetoin yield, etc., and achieve the effects of less impurities, less side reactions, and less poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
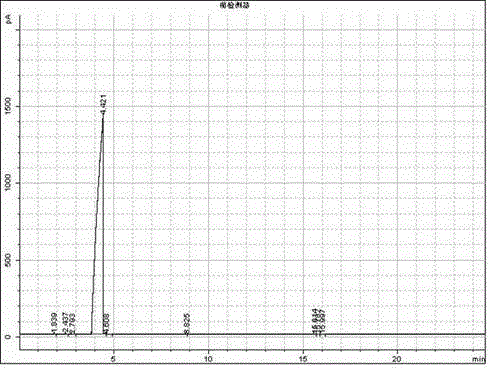
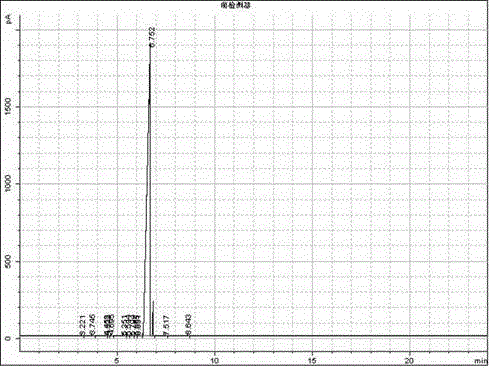
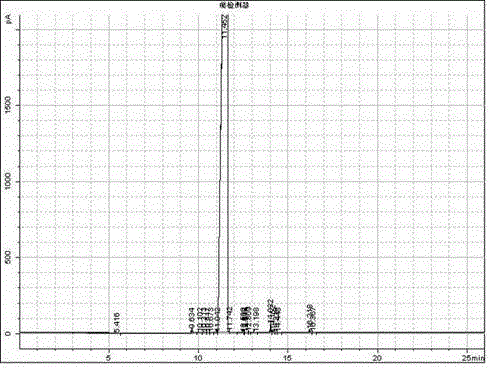
Image
Examples
Embodiment 1
[0023] A preparation method of acetoin compounds, the steps are: step 1: under stirring conditions, add aldehydes and catalysts with a weight ratio of (100-200): 1 to the reaction kettle, the aldehydes are acetaldehyde, propionaldehyde or Butyraldehyde, the reaction temperature is controlled at 70-120°C, the reaction pressure is 0.4-0.8MPa, the reaction time is 0.5-5h, and the reaction solution is obtained; Step 2: The reaction solution in step 1 is subjected to vacuum distillation, and the distillation temperature is collected at 80 The distillate between ~160°C is the azoin compound, and the atoin compound is acetoin, propioin or butyroin.
[0024] Above-mentioned catalyst is 1-n-butylbenzimidazole hydrochloride, and its structural formula is The preparation steps of the catalyst are as follows: put benzimidazole, chlorobutane and acetonitrile with a weight ratio of 1:(1~5):(2~20) in a three-necked flask, heat to reflux, the reflux temperature is 80°C, and reflux The time ...
Embodiment 2
[0027] (1) Preparation of catalyst
[0028] Get 36g of benzimidazole, 108g of chlorobutane and 360g of acetonitrile in a three-necked flask, the weight ratio of benzimidazole, chlorobutane and acetonitrile is 1:3:10, heat to reflux, and the reflux temperature is 100°C , Reflux 10h, obtain catalyst crude material, then catalyst crude material is through centrifugation, washing, drying the 1-n-butylbenzimidazole hydrochloride that obtains 143g, and its proton nuclear magnetic resonance spectrum is: 1 H NMR (CDCl 3 ):0.90(t,J=7.4Hz,3H),1.31(m,2H),1.74(m,2H),4.04(t,J=7.5Hz,2H),7.22(m,2H),7.59(m ,2H), 8.26(s,1H).
[0029] The reaction equation for preparing the catalyst is:
[0030]
[0031] (2) Preparation of Acetoin
[0032] Under stirring condition, add the acetaldehyde of 500g and the 1-n-butylbenzimidazole hydrochloride of 2.5g in reactor, the weight ratio of acetaldehyde and 1-n-butylbenzimidazole hydrochloride is 200: 1. The reaction temperature is controlled at 80°C...
Embodiment 3
[0036] (1) Preparation of catalyst
[0037] Get 36g of benzimidazole, 108g of chlorobutane and 360g of acetonitrile in a three-necked flask, the weight ratio of benzimidazole, chlorobutane and acetonitrile is 1:3:10, heat to reflux, and the reflux temperature is 100°C , Reflux 10h, obtain catalyst crude material, then catalyst crude material is through centrifugation, washing, drying the 1-n-butylbenzimidazole hydrochloride that obtains 143g, and its proton nuclear magnetic resonance spectrum is: 1 H NMR (CDCl 3 ):0.90(t,J=7.4Hz,3H),1.31(m,2H),1.74(m,2H),4.04(t,J=7.5Hz,2H),7.22(m,2H),7.59(m ,2H), 8.26(s,1H).
[0038] The reaction equation for preparing the catalyst is:
[0039]
[0040] (2) Preparation of Propionin
[0041] Under stirring condition, add the N-butylbenzimidazole hydrochloride of 500g propionaldehyde and 3.5g in the reactor, the weight ratio of propionaldehyde and 1-n-butylbenzimidazole hydrochloride is 143:1 , the reaction temperature is controlled at 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com