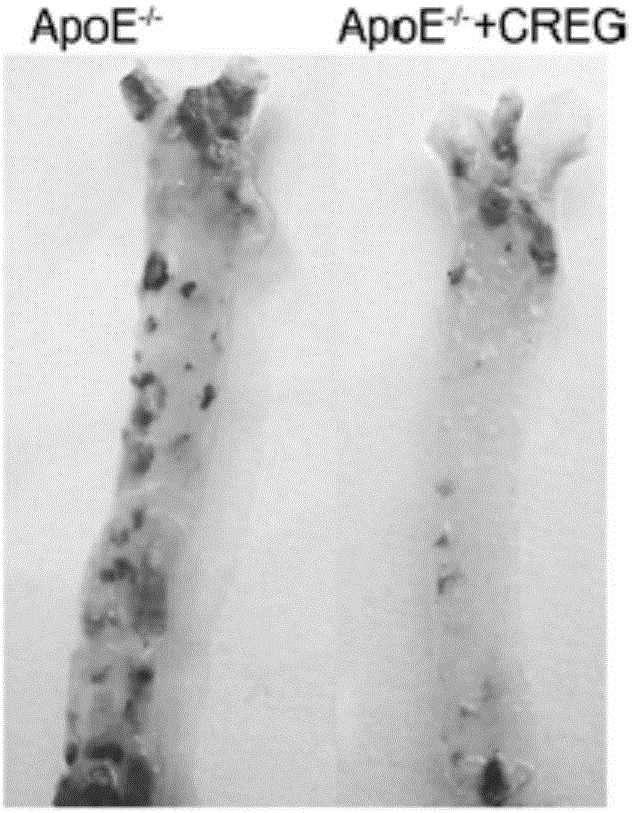
Novel application of recombinant hCREG glycoprotein and application thereof on coronary stent
A glycoprotein, coronary artery technology, applied to the new use of recombinant hCREG glycoprotein, the application field of coronary stent, can solve the problem of limited research work on CREG protein, to promote endothelial cell repair and growth, inhibit atherosclerosis , the effect of solving restenosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 recombinant hCREG glycoprotein
[0052] The preparation method of described recombinant hCREG glycoprotein comprises the following steps:
[0053] (1) Construction of the recombinant gene vector: RT-PCR technique was used to amplify the hCREG open reading frame of the stop codon mutation, and the hCREG RT-PCR primers of the stop codon mutation were as follows: the upstream primer was 5'-aa ggatcc atggccgggctatcccgc-3'( SEQ ID NO.2), the downstream primer is: 5'-gc gaattc Gcactgaactgtgacatttaatattcttctgg-3' (SEQ ID NO.3), wherein. Capital letters represent the stop codon of C / G mutation, construct the recombinant human CREG protein expression vector pcDNA3.1-his / myc-hCREG with his tag protein, and the recombinant hCREG glycoprotein contains as shown in SEQ ID NO.1 The amino acid sequence of is as follows:
[0054] MAGLSRGSARALLAALLASTLLALLVSPARGRGGRDHGDWDEASRLPPLPPREDAARVARFVTHVSDWGALATISTLEAVRGRPFADVLSLSDGPPGAGSGVPYFYLSPLQLSVSNLQENPYATL...
Embodiment 2
[0058] Example 2 Preparation of recombinant hCREG glycoprotein coronary stent
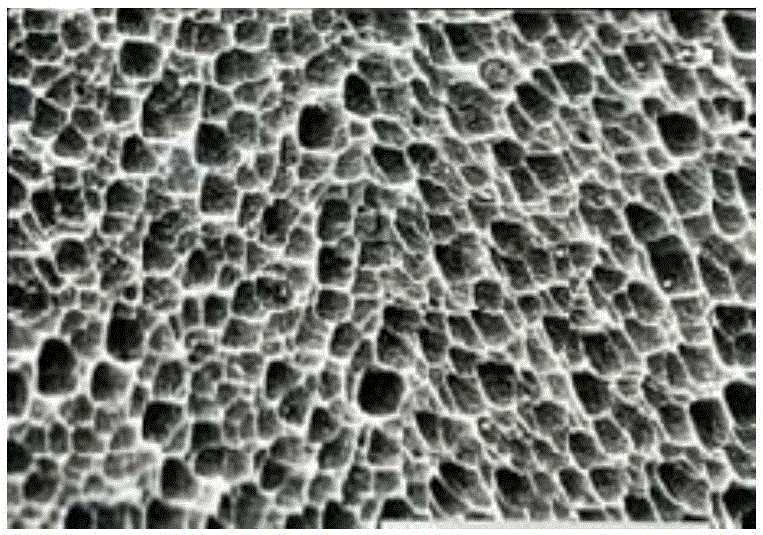
[0059] The nano-microporous bare metal stent of Lepu (Beijing) Medical Devices Co., Ltd. was used. The 316L stainless steel bare metal stent was corroded with 38% hydrochloric acid for 12 hours and then corroded with 28% hydrochloric acid for 5 minutes under the action of an electric field. Nanopores of 100-800nm are prepared on the surface of the scaffold.
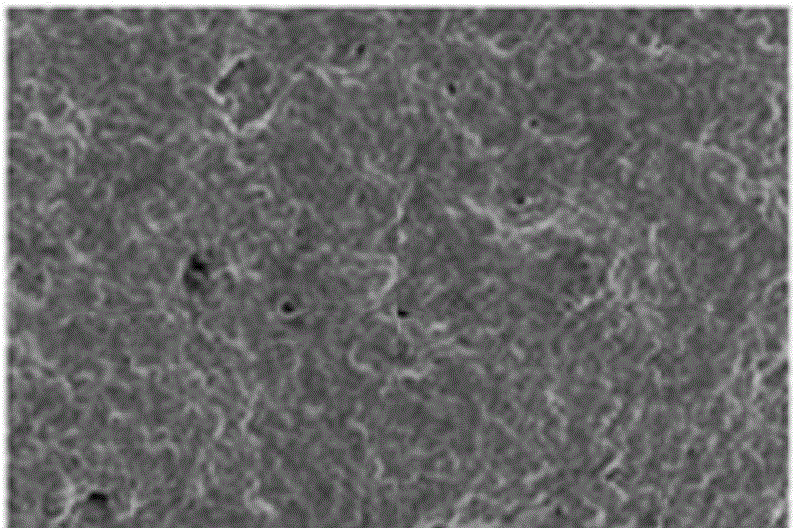
[0060] Using the CREG protein prepared in Example 1, use a sodium carbonate buffer solution with a pH of 9.6 (0.05mol / L) to prepare the CREG protein into a coating buffer solution of 1.6 mg / mL, and put the nanoporous bare metal stent into the preparation The good coating buffer solution was taken out after soaking for 48 hours, freeze-dried in a vacuum dryer at -55°C, and stored at 4°C.
[0061] The scanning electron micrograph of the prepared recombinant hCREG glycoprotein coronary stent is shown in FIG. 1 , and it can be seen that a large...
Embodiment 3
[0062] Example 3 Preparation of drug-protein combined scaffold
[0063] Nanoporous bare metal stents were prepared according to the method in Example 2.
[0064] Prepare rapamycin drug with a concentration of 1% in acetone solution. A balloon is used to protect the inner surface of the stent, and rapamycin drug is sprayed on the outer surface of the stent.
[0065] The sodium carbonate buffer solution with pH of 9.6 (0.05mol / L) was used to prepare CREG glycoprotein into a coating buffer of 1.6 mg / mL, put the nanoporous bare metal stent into the prepared coating buffer, soak for 48 After 1 hour, it was taken out, freeze-dried in a vacuum dryer at -55°C, and stored at 4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com