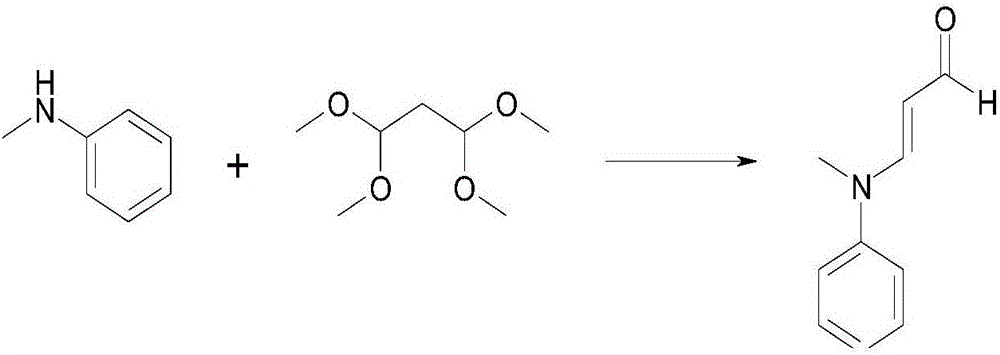
Preparation method of 3-(N-methyl-N-phenyl)aminoacrolein
A technology of aminoacrolein and methylaniline, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of high raw material prices, long reaction time, cumbersome post-processing, etc., and reduce the generation of impurities , improve quality, and reduce the effect of three wastes pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Put 100g of tetramethoxypropane and 58.8g of N-methylaniline in a 1L reaction flask in turn, stir for 10 minutes, control the internal temperature below 30°C, add 22g of 20% hydrochloric acid dropwise, keep the temperature at 24°C and stir for 2.5 hours. Add 200 g of toluene, add 20% liquid caustic soda to control the temperature below 30°C to make the pH of the water layer 6-7, stand still for layering, extract the water layer with toluene, combine the organic layers, wash three times with saturated brine, stand still to separate the water layer. The organic layer is first reduced in pressure to recover the solvent, and then the 60-70°C / 40mmHg fraction is collected to obtain 83.1 g of 3-(N-methyl-N-phenyl)amino acrolein with a content of 99.6%.
Embodiment 2
[0027] Put 100g of tetramethoxypropane and 62g of N-methylaniline in a 1L reaction flask in sequence, stir for 10 minutes, control the internal temperature below 30℃, add 22g of 20% hydrochloric acid dropwise, keep warm and stir for 2.5 hours at about 25℃, add 200g of toluene, add 20% liquid caustic soda to control the temperature below 30℃ to make the pH of the water layer 6~7, stand still for layering, extract the water layer with toluene, combine the organic layers, wash three times with saturated brine, stand still to separate the water layer, organic The layer was firstly decompressed to recover the solvent, and then the 60-70°C / 40mmHg fraction was collected to obtain 83.5g of 3-(N-methyl-N-phenyl)amino acrolein with a 99.5% content.
Embodiment 3
[0029] Put 100g of tetramethoxypropane and 65.4g of N-methylaniline in a 1L reaction flask in turn, stir for 10 minutes, control the internal temperature below 30°C, add 22g of 20% hydrochloric acid dropwise, keep the temperature at 26°C and stir for 2.5 hours. Add 200 g of toluene, add 20% liquid caustic soda to control the temperature below 30°C to make the pH of the water layer 6-7, stand still for layering, extract the water layer with toluene, combine the organic layers, wash three times with saturated brine, stand still to separate the water layer. The organic layer is firstly decompressed to recover the solvent, and then the 60-70° C. / 40 mmHg fraction is collected to obtain 83.4 g of 3-(N-methyl-N-phenyl)amino acrolein with a content of 99.5%.
[0030] Example 2 is a preferred trial method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com