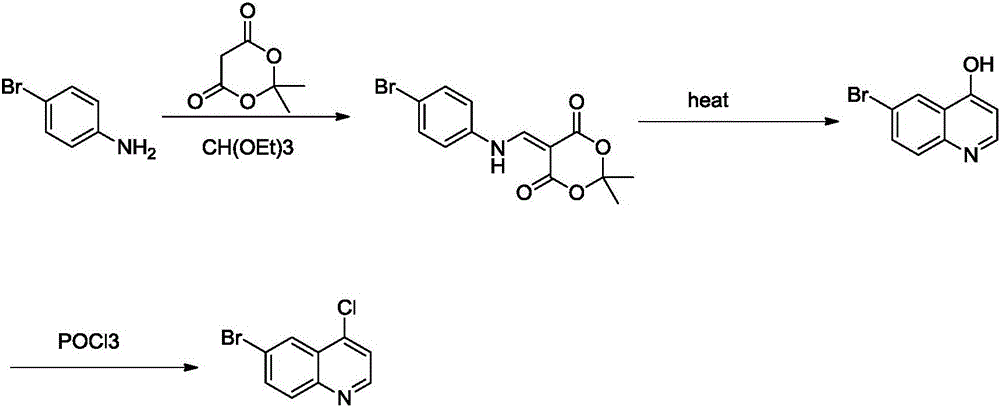
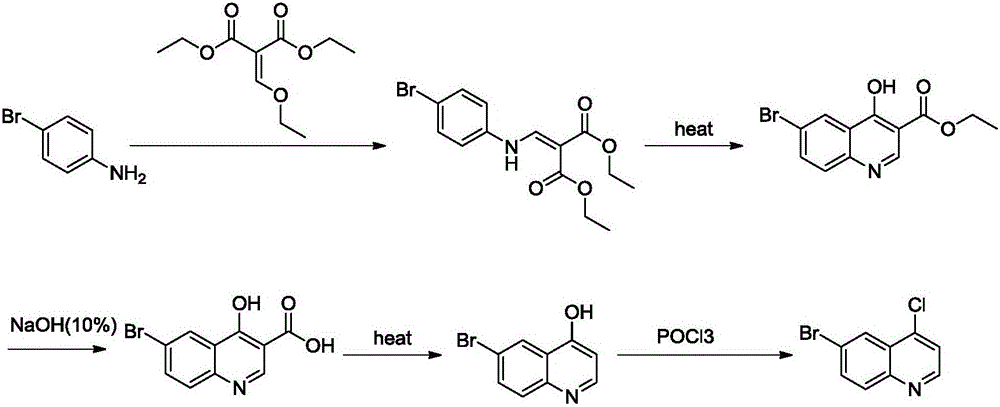
6-bromo-4-chloroquinoline preparation method
A technology of chloroquinoline and bromoquinoline, applied in the field of medicinal chemistry, can solve the problems of long process steps, difficult reactions, complicated operations, etc., and achieve the effects of simplifying the reaction process and post-processing process, reducing production costs, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
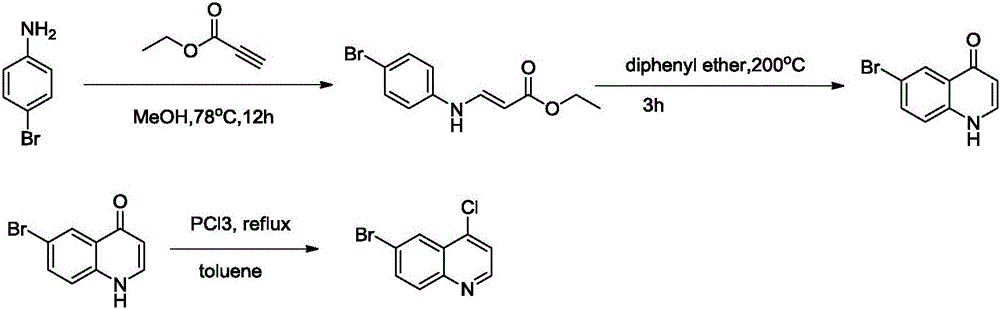
[0022] The first step: the synthesis of 3-(4-bromoaniline) ethyl acrylate
[0023] 28.51g (290.66mmol) of ethyl propiolate was added to a 1L three-necked flask containing 50g (290.66mol) of 4-bromoaniline and methanol (500ml) under nitrogen protection with stirring, heated to 40°C, and stirred for 48h , TLC detected that after the reaction was completed, the solvent was spin-dried to obtain 3-(4-bromoaniline) ethyl acrylate (78 g, yield 99%) as a crude product which was directly used in the next step.
[0024] The second step: the synthesis of 6-bromoquinolin-4(1H)-one
[0025] Dissolve 78 g of crude 3-(4-bromoaniline) ethyl acrylate in 150 ml of diphenyl ether, and slowly drop it into a 1L three-necked bottle containing diphenyl ether (470 ml) at 200°C. After 2 hours of reaction, the spot of starting material disappeared. The reaction solution was cooled to room temperature, poured into 1500 ml of petroleum ether, left standing overnight and filtered, the filter residue was...
Embodiment 2
[0032] The first step: the synthesis of 3-(4-bromoaniline) ethyl acrylate
[0033] 57.02g (581.32mmol) of ethyl propiolate was added to a 1L three-necked flask containing 50g (290.66mol) of 4-bromoaniline and methanol (500ml) under nitrogen protection with stirring, heated to 30°C, and stirred for 72h , TLC detected that after the reaction was completed, the solvent was spin-dried to obtain 3-(4-bromoaniline) ethyl acrylate (80 g, yield 100%) as a crude product which was directly used in the next step.
[0034] The second step: the synthesis of 6-bromoquinolin-4(1H)-one
[0035] Dissolve 80 g of crude 3-(4-bromoaniline) ethyl acrylate in 150 ml of diphenyl ether, and slowly drop it into a 1L three-necked bottle containing diphenyl ether (400 ml) at 220°C. After 10 hours of reaction, the starting point of the spot plate disappeared. The reaction solution was cooled to room temperature, poured into 1500 ml of petroleum ether, left standing overnight and filtered, the filter re...
Embodiment 3
[0041] The first step: the synthesis of 3-(4-bromoaniline) ethyl acrylate
[0042] 42.76g (435.99mmol) of ethyl propiolate was added to a 1L three-necked flask containing 50g (290.66mol) of 4-bromoaniline and methanol (500ml) under nitrogen protection with stirring, heated to 50°C, and stirred for 32h. , TLC detected that after the reaction was completed, the solvent was spin-dried to obtain 3-(4-bromoaniline) ethyl acrylate (80 g, yield 100%) as a crude product which was directly used in the next step.
[0043] The second step: the synthesis of 6-bromoquinolin-4(1H)-one
[0044] Dissolve 80 g of crude 3-(4-bromoaniline) ethyl acrylate in 150 ml of diphenyl ether, and slowly drop it into a 1L three-necked bottle containing diphenyl ether (250 ml) at 220°C. After 2 hours of reaction, the spot of starting material disappeared. The reaction solution was cooled to room temperature, poured into 1500 ml of petroleum ether, left to stand overnight, and filtered. The filter residue ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com