Fused ring compound with four-nitrogen and nine-ring structure and preparation method of fused ring compound
A technology of benzo and imidazo, which is applied in the field of organic photoelectric materials, can solve the problems of difficult separation and purification, cumbersome preparation routes, and harsh conditions, and achieve the effect of short steps, simple operation, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1. 5,6-Dibutylbenzo[4',5']isoquinoline[2',1':1,2]imidazo[4,5-c]indole[2,3- a] Preparation of (3a) of carbazol-18-one
[0019]
[0020] 5,6-Dibutylbenzo[4',5']isoquinoline[2',1':1,2]imidazo[4,5-c]indole[2,3-a]carbazole -18-ketone (3a): Add 11,12-di-n-butyl-indole[2,3-a]carbazole-5,6-dione (1) (0.40g, 1mmol), acenaphthoquinone (5a) (0.18g, 1mmol), ammonium formate (0.13g, 2mmol), ammonium acetate (0.15g, 2mmol), ethanol 5mL, the condenser tube was sealed with a balloon, the reaction system was heated to a slight boil, and the reaction After 6 hours, the reaction system was cooled to room temperature, added an appropriate amount of water and stirred for 15 minutes and extracted with chloroform, the organic phases were combined, dried over anhydrous magnesium sulfate, filtered to remove insoluble matter, and the filtrate was concentrated and purified by column chromatography (V 氯仿 :V 正己烷 =30:70), and finally obtained 0.42 g of brown-red solid with a yield of 7...
Embodiment 2
[0021] Example 2. 5,6-Dibutylbenzo[4',5']isoquinoline[2',1':1,2]imidazo[4,5-c]indole[2,3- a] Preparation of (3a) of carbazol-18-one
[0022]
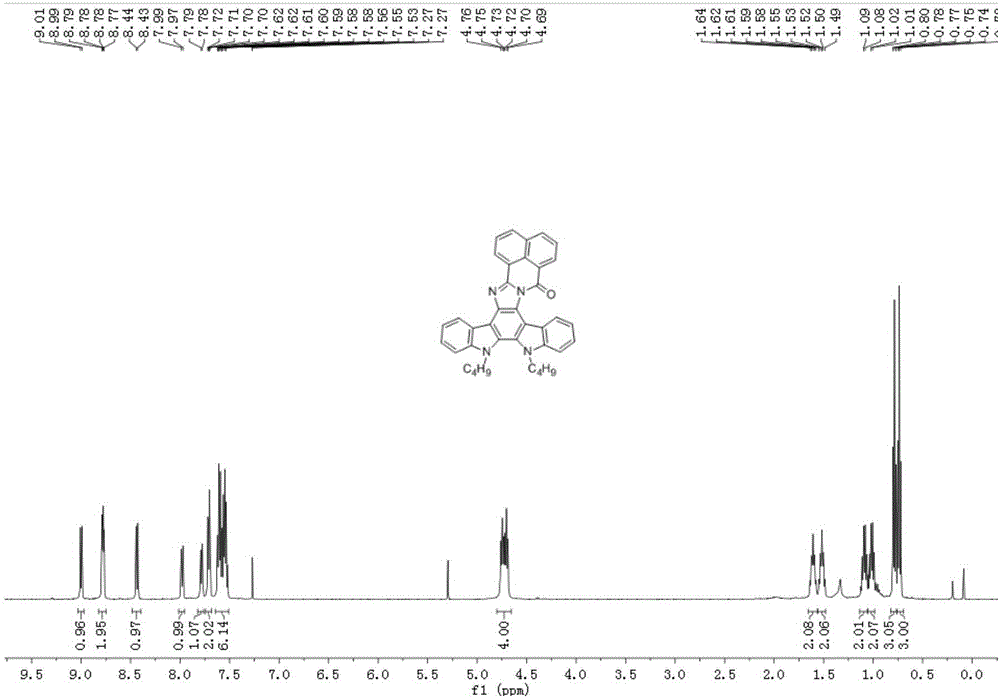
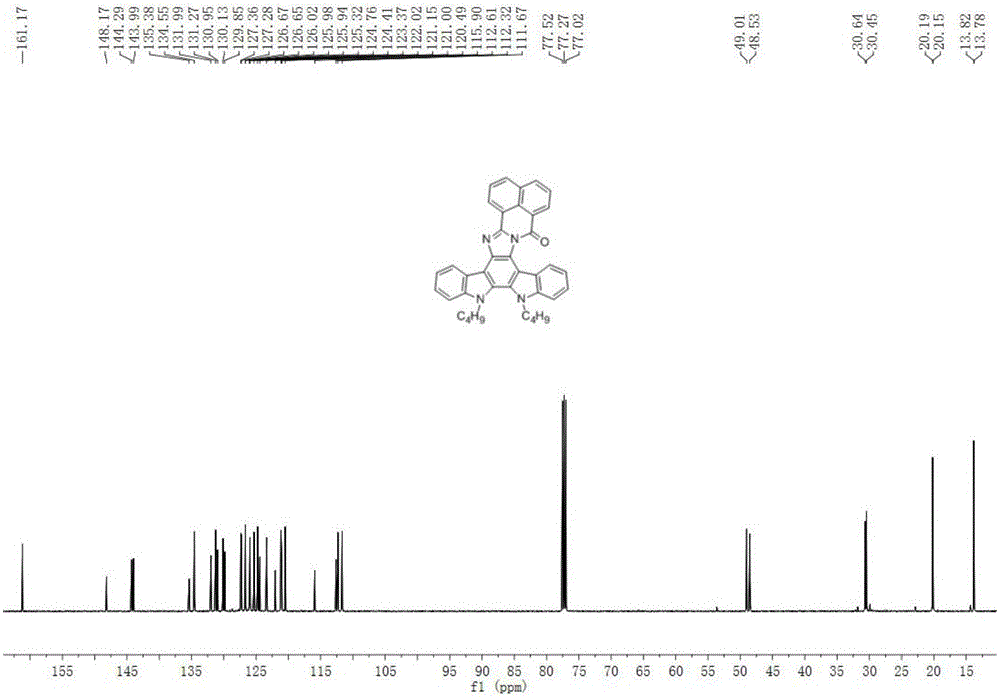
[0023] 5,6-Dibutylbenzo[4',5']isoquinoline[2',1':1,2]imidazo[4,5-c]indole[2,3-a]carbazole -18-ketone (3a): Add 11,12-di-n-butyl-indole[2,3-a]carbazole-5,6-dione (1) (0.80g, 2mmol), acenaphthoquinone (5a) (0.72g, 4mmol), ammonium chloride (0.42g, 8mmol), ammonium acetate (0.62g, 8mmol), toluene 3mL, N,N-dimethylformamide 3mL, for condenser Seal the balloon, heat the reaction system to a slight boil, react for 8 hours, cool the reaction system to room temperature, add an appropriate amount of water and extract with chloroform, combine the organic phases, dry over anhydrous magnesium sulfate, remove insoluble matter by filtration, and concentrate the filtrate through the column Chromatographic purification (V 氯仿 :V 正己烷 =30:70), and finally obtained 0.89 g of brown-red solid with a yield of 80%. 1 H NMR (500MHz, CDCl 3 )δ9.00(d, J=...
Embodiment 3
[0024] Example 3. 5,6-Dibutylbenzo[4',5']isoquinoline[2',1':1,2]imidazo[4,5-c]indole[2,3- a] Preparation of (3a) of carbazol-18-one
[0025]
[0026] 5,6-Dibutylbenzo[4',5']isoquinoline[2',1':1,2]imidazo[4,5-c]indole[2,3-a]carbazole -18-ketone (3a): Add 11,12-di-n-butyl-indole[2,3-a]carbazole-5,6-dione (1) (0.80g, 2mmol), acenaphthylquinone (5a) (0.54g, 3mmol), ammonium acetate (1.82g, 20mmol), acetic acid 5mL, the condenser tube was sealed with a balloon, the reaction system was heated to a slight boil, reacted for 10 hours, and the reaction system was cooled to room temperature After adding an appropriate amount of water, extract with chloroform, the organic phases are combined, dried over anhydrous magnesium sulfate, filtered to remove insoluble matter, and the filtrate is concentrated and purified by column chromatography (V 氯仿 :V 正己烷 =30:70), 1.00 g of a brown-red solid was finally obtained, with a yield of 90%. 1 H NMR (500MHz, CDCl 3 )δ9.00(d, J=7.6Hz, 1H), 8.80–8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com