Diphenylacetypene liquid crystal molecule containing pyridine end group as well as preparation method thereof and application thereof
A technology of diphenylacetylene and liquid crystal molecules, which is applied in liquid crystal materials, chemical instruments and methods, photosensitive equipment, etc., to achieve the effect of broadening the application range, high clearing point and good compatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
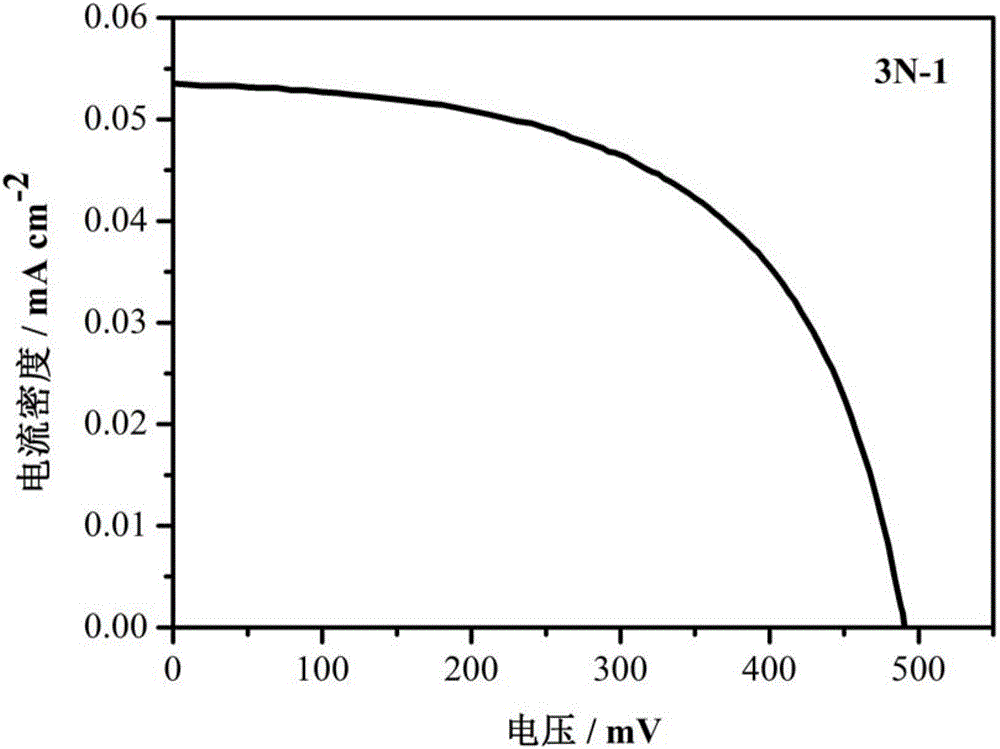
[0063] Synthesize the tolan liquid crystal molecule 3N-1 containing pyridine terminal group, the specific synthesis method is as follows:
[0064] 1. Synthesis of compound 1
[0065]
[0066] in N 2 Under protection, 1.04g (8.46mmol) 4-pyridine boronic acid, 2.83g (10.00mmol) p-bromoiodobenzene, 3.50g (25.38mmol) potassium carbonate, 10mL H 2 O and 50mL of DMF were added to a 100mL three-neck flask equipped with a thermometer, a condenser, and a magnetic stirrer, and stirred at 40°C for 30 minutes, then the temperature was raised to 60°C, and then 0.28g (0.24mmol) of tetrakis(triphenyl)phosphine palladium was added , continue the reaction at 60°C for 15 hours, cool the reaction solution to room temperature naturally, extract the reaction solution three times with dichloromethane, and finally combine the organic phase, wash the organic phase with brine three times, dry it with anhydrous magnesium sulfate for half an hour, and filter it with suction The solvent was recovere...
Embodiment 2
[0080] Synthesize the tolan liquid crystal molecule 3FN-1 containing pyridine terminal group, the specific synthesis method is as follows:
[0081] 1. Synthesis of Compound 1
[0082] Compound 1 was prepared according to the method in Step 1 of Example 1.
[0083] 2. Synthesis of the tolan liquid crystal molecule 3FN-1 containing a pyridine terminal group
[0084]
[0085] in N 2 Under protection, 3.09g (9.36mmol) of fluorine-containing aromatic alkyne alcohol 3FOH, 0.25g (0.78mmol) of tetra-n-butylammonium bromide, 5.24g (93.6mmol) of potassium hydroxide, 16mL of toluene and 4mL of distilled water were added to the In a three-necked flask with a thermometer, a magnetic stirrer, and a condenser, place the three-necked flask in an oil bath, and heat up to 60°C for 40 minutes of reaction. After the solid is completely dissolved, add 1.83g (7.8mmol) of compound 1 and 0.27 g (0.23mmol) tetrakis(triphenyl)phosphine palladium, heated to 80°C, stirred at constant temperature fo...
Embodiment 3
[0090]Synthesize the tolan liquid crystal molecule 3F2N-1 containing pyridine terminal group, the specific synthesis method is as follows:
[0091] 1. Synthesis of Compound 4
[0092]
[0093] In Step 1 of Example 1, the p-bromoiodobenzene used was replaced with equimolar 3-fluoro-4-bromoiodobenzene, and other steps were the same as Step 1 of Example 1 to obtain Compound 4.
[0094] 2. Synthesis of tolan liquid crystal molecule 3F2N-1 containing pyridine terminal group
[0095]
[0096] In Step 2 of Example 2, the compound 1 used was replaced by an equimolar compound 4, and the other steps were the same as Step 2 of Example 2 to obtain the tolan liquid crystal molecule 3F2N-1 containing a pyridine terminal group. The data confirmed by the nuclear magnetic structure shows that it is the target product, and its liquid crystal phase transition performance is not substantially different from that of Example 2, and will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com