Liquid crystal compound containing acetal ring and preparation method thereof
A liquid crystal compound and compound technology, applied in chemical instruments and methods, liquid crystal materials, organic chemistry, etc., can solve the problem that liquid crystal monomers cannot meet the needs of liquid crystal display, etc., to improve the light response speed, broaden the application range, and clear the point. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Under nitrogen protection at a flow rate of 0.6mL / min, 2.55g of 2-methyl-4-(4-trans-(4-n-propylcyclohexyl)ethylphenyl)-3-butyn-2-ol , 0.25g tetra-n-butylammonium bromide, 5.23g potassium hydroxide, 16mL toluene and 4mL distilled water are added in the three-necked flask that thermometer, magnetic stirring bar, condenser tube are housed, and the three-necked flask is placed in the oil bath, and Raise the temperature to 60°C and react for 40 minutes. After the solid is completely dissolved, add 2.00g of 4-bromophenylpropionaldehyde acetal and 0.27g of tetrakis(triphenyl)phosphine palladium, raise the temperature to 80°C, and stir the reaction at constant temperature for 12 hours to end the reaction , the reaction solution was cooled to room temperature, and filtered with diatomaceous earth, the filtrate was extracted with ethyl acetate, and the organic phase obtained after liquid separation was washed to neutrality, then dried with anhydrous magnesium sulfate and concentra...
Embodiment 2
[0043] In Example 1, the 4-bromophenylpropionaldehyde acetal used was replaced with an equimolar 3-fluoro-4-bromophenylpropionaldehyde acetal, and the other steps were the same as in Example 1 to obtain white crystals—containing acetal The liquid crystal compound of ring, its yield is 50%, and chemical name is 1-{4-[2-(4-n-propylcyclohexyl) ethyl] phenyl}-2-[2-fluoro-4-(propane Aldehyde (ethylene glycol acetal) phenyl] acetylene, the specific chemical reaction equation is as follows:
[0044]
[0045] The structural characterization data of the resulting white crystals are as follows:
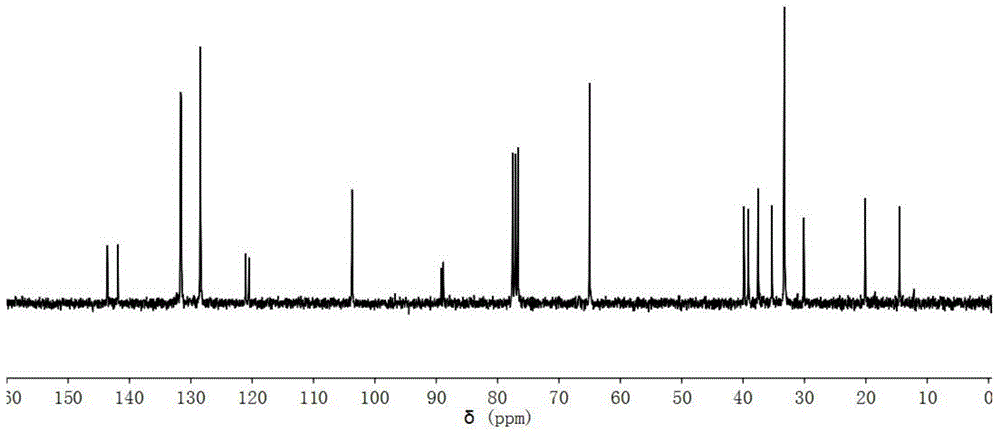
[0046] 13 C-NMR (CDCl 3 as solvent, internal standard is TMS, 75MHz, ppm): 164.2, 160.9, 144.6, 144.0, 133.2, 131.6, 128.4, 124.1, 120.2, 115.3, 109.6, 103.5, 94.2, 82.2, 65.0, 39.8, 39.1, 37.5, 37.5 , 35.0, 33.3, 33.3, 33.3, 29.9, 20.1, 14.5. Spectrum such as Figure 4 shown.
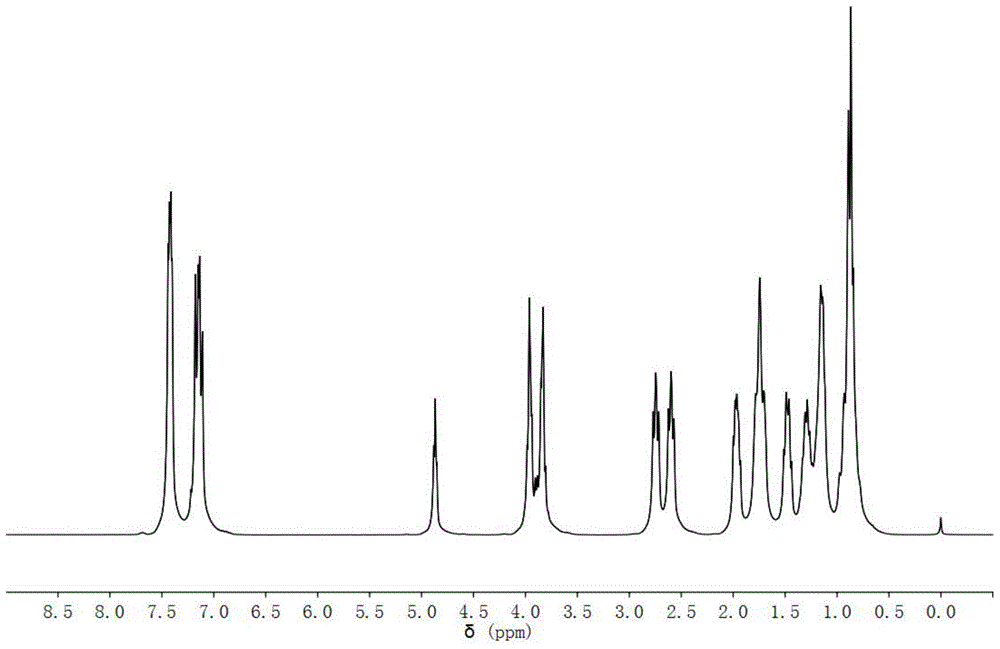
[0047] 1 H-NMR (CDCl 3 As a solvent, the internal standard is TMS, 300MHz, ppm): 7.40 (dd, J = 18.0, 7.8Hz...
Embodiment 3
[0052] In Example 1, the 2-methyl-4-(4-trans-(4-n-propylcyclohexyl)ethylphenyl)-3-butyn-2-alcohol was used with equimolar 2-methyl Base-4-(4-trans-(4-n-ethylcyclohexyl)ethylphenyl)-3-butyn-2-alcohol was replaced, and other steps were the same as in Example 1 to obtain white crystals—containing acetal The liquid crystal compound of ring, its yield is 45%, and chemical name is 1-{4-[2-(4-n-ethylcyclohexyl) ethyl] phenyl}-2-[4-(propionaldehyde ethylene glycol Acetal) phenyl] acetylene, the specific chemical reaction equation is as follows:
[0053]
[0054] The NMR structure confirmation data showed that the obtained white crystal was the target product, and its optical anisotropy and dielectric anisotropy properties were not substantially different from those of the liquid crystal compound obtained in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com