A kind of thiophene liquid crystal molecule containing pyridine terminal group and its preparation method and application
A liquid crystal molecule and thiophene-based technology, which is applied in the direction of liquid crystal materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., to achieve the effect of broadening the application range and high clearing point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of thiophene liquid crystal molecule 3SN containing pyridine terminal group, the specific synthesis route and method are as follows:
[0048] 1. Synthesis of Compound 1
[0049]
[0050] in N 2 Under protection, 1.9g (8.1mmol) dibromothiophene, 1.0g (8.14mmol) 4-pyridineboronic acid, 2.2g (16.3mmol) potassium carbonate, 50mL THF , 10mL of water, stirred at 40°C for 30 minutes and then warmed up to 60°C, then added 0.2758g (0.24mmol) tetrakis(triphenyl)phosphine palladium, stirred at 60°C for 15 hours, extracted the reaction solution with dichloromethane, and the organic phase After drying over anhydrous magnesium sulfate, filtering, and concentrating, the crude product was obtained, and the crude product was separated and purified by column chromatography (with a mixture of petroleum ether and ethyl acetate at a volume ratio of 5:1 as the eluent) to obtain a yellow solid Compound 1, its yield is 45%.
[0051] 2. Synthesis of target compound 3SN
[0052]...
Embodiment 2
[0058] Synthesis of thiophene liquid crystal molecule 3FSN containing pyridine terminal group, the specific synthesis method is as follows:
[0059] 1. Synthesis of compound 1
[0060] Compound 1 was prepared according to the method in Step 1 of Example 1.
[0061] 2. Synthesis of target compound 3FSN
[0062]
[0063] In step 2 of embodiment 1, the compound 3OH used is replaced with equimolar compound 3FOH, and other steps are the same as step 2 of embodiment 1 to obtain white crystals——containing pyridine terminal group thiophene liquid crystal molecules 3FSN, and the yield is 45%, HPLC purity 99.40%, structural characterization data are as follows:
[0064] 13 C-NMR (CDCl 3 is the solvent, the internal standard is TMS, 101MHz, ppm): 163.66, 161.16, 150.43, 147.10, 140.57, 133.02, 125.27, 124.81, 124.16, 124.13, 119.66, 115.37, 108.12, 88.73, 86.31, 38.69 , 33.21, 33.13, 33.10, 19.98, 14.37.
[0065] 1 H-NMR (CDCl 3 is the solvent, the internal standard is TMS, 40...
Embodiment 3
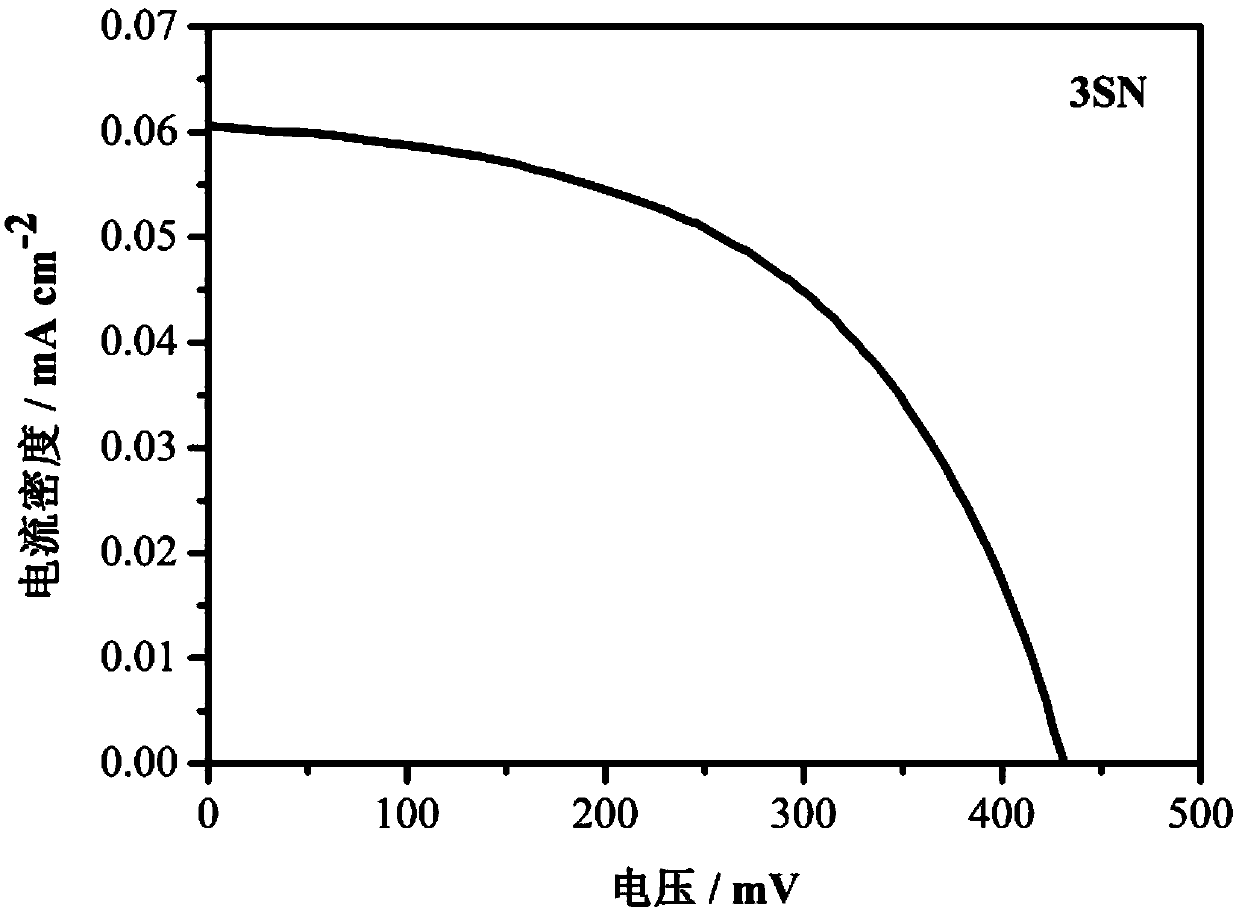
[0068] Use of the thiophene liquid crystal molecule 3SN containing pyridine terminal groups prepared in Example 1 in the preparation of dye-sensitized solar cells.
[0069] Its specific usage method is:
[0070] 1. Conductive glass pretreatment
[0071] Clean the conductive glass in detergent, ethanol, and deionized water with an ultrasonic wave at a frequency of 40 Hz and a power of 100 W for 30 minutes to 1 hour, and dry it at 110°C for later use.
[0072] 2. Preparation of dye solution
[0073] Dissolve thiophene liquid crystal molecules containing pyridine terminal groups in a mixed solvent of acetonitrile and tert-butanol at a volume ratio of 1:1 to prepare a dye solution with a concentration of 0.3 mmol / L.
[0074] 3. Preparation of electrolyte solution
[0075] Add tetrabutylammonium iodide, lithium iodide, iodine, and p-tert-butylpyridine into acetonitrile to prepare an electrolyte solution. The concentrations are 0.6mol / L, 0.1mol / L, 0.05mol / L, and 0.5mol / L, respec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com