Method for in-situ control of hydrogen sulfide odor in sediments
A sediment and hydrogen sulfide technology, which is applied in the oxidation treatment of sludge, waterway systems, sewer pipeline systems, etc., can solve the problems of large amount of engineering, expensive pipeline dredging, inapplicability of interception and sewage law enforcement, etc., and achieves low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add granular iron hydroxide (GFH) to the water containing hydrogen sulfide, so that the molar ratio of iron and hydrogen sulfide in the water is greater than 1:1, and then fully mix the mixed solution for 1 to 24 hours to complete the hydrogen sulfide removal. The water used is the water sample containing hydrogen sulfide prepared in the laboratory. At room temperature (22±1°C), NaHCO 3 , HCl and NaOH to adjust the pH to 7.1, then add GFH to the water containing hydrogen sulfide, so that the ratio of hydrogen sulfide to hydrogen sulfide is 2:1 and 8:1 respectively, and the reactor is a 43mL airtight plastic bottle. The reactor was placed on a shaker and mixed thoroughly (200rpm), and samples were taken at regular intervals to observe the removal effect of hydrogen sulfide.
[0046] Figure 4 It is a figure showing the removal effect of hydrogen sulfide in this embodiment. As shown in the figure, the removal of hydrogen sulfide with GFH is effective and feasible, and ...
Embodiment 2
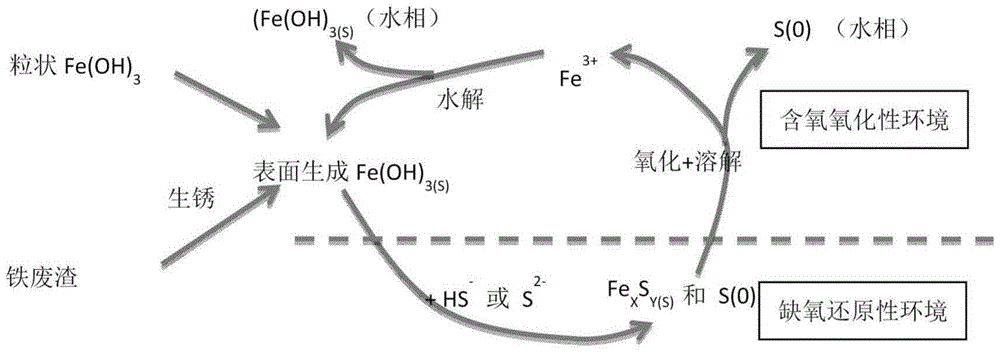
[0048] Add granular iron oxide, iron hydroxide, zero-valent iron or iron slag to the sediment of rainwater pipes, rivers or sewage pipes, and mix evenly to achieve complete removal of hydrogen sulfide. When the effect of granular iron in controlling hydrogen sulfide declines, set a gate at the connection between the rainwater pipe and the seawater inlet and close the gate, so that the seawater high tide level reaches a certain water level (1.0-2.6m), and then open the gate to enrich the oxygen The seawater forms a turbulent flow, fully contacts with the sediment and the invalid granular iron, and the regenerated iron particles co-precipitate with the sediment. The above regeneration process can be repeated several times as required until the desired regeneration effect is achieved.
[0049] Figure 5It is a figure showing the removal effect of hydrogen sulfide in the present embodiment, showing the effect of using GFH to control the generation of hydrogen sulfide from deposit...
Embodiment 3
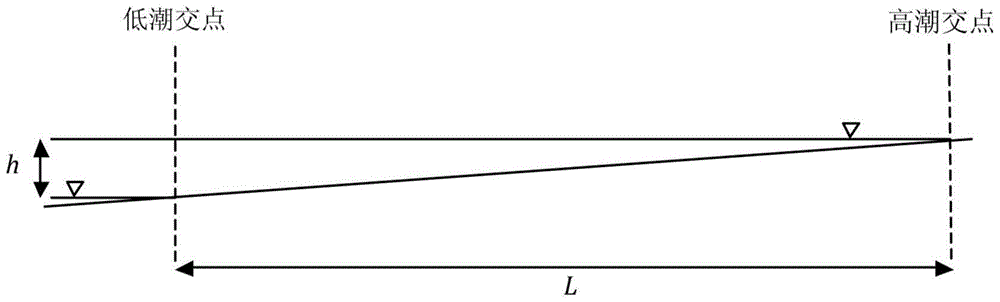
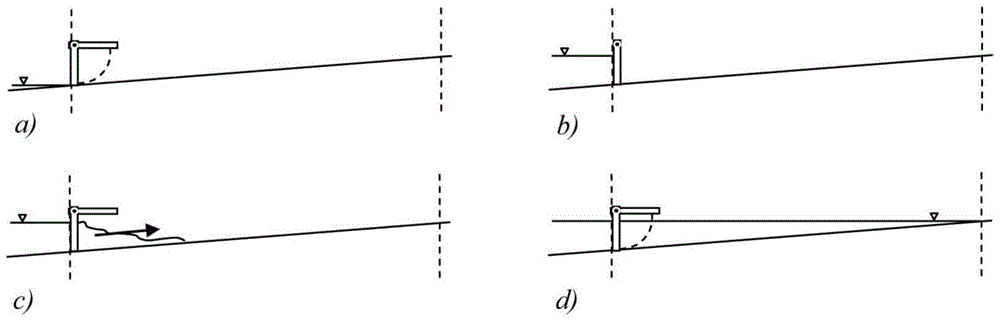
[0052] Mix GFH evenly into the artificial bottom mud (clean quartz sand with a particle size of 0.5-1.0 mm), so that the concentration of GFH in the bottom mud is 2.18 grams per liter of sediment, and then fill the mixture into a columnar reactor, and continuously The GFH is deactivated by passing an aqueous solution containing hydrogen sulfide, so that the hydrogen sulfide removal capacity of the GFH can be calculated based on the total amount of hydrogen sulfide removed. Sediment containing failed GFH was spread flat in a small storm sewer simulator (e.g. Figure 6 As shown, the slope is 1:20, the width is 0.3m), and the thickness of the bottom mud layer is about 10mm. The end of the rainwater pipe is connected to a reservoir filled with water. Gates, by opening the gates quickly to create the hydraulic flushing effect mentioned above, allowing the oxygen-enriched water to mix well with the sediment. Since the volume of the cistern is about 18 times that of the rainwater pi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com