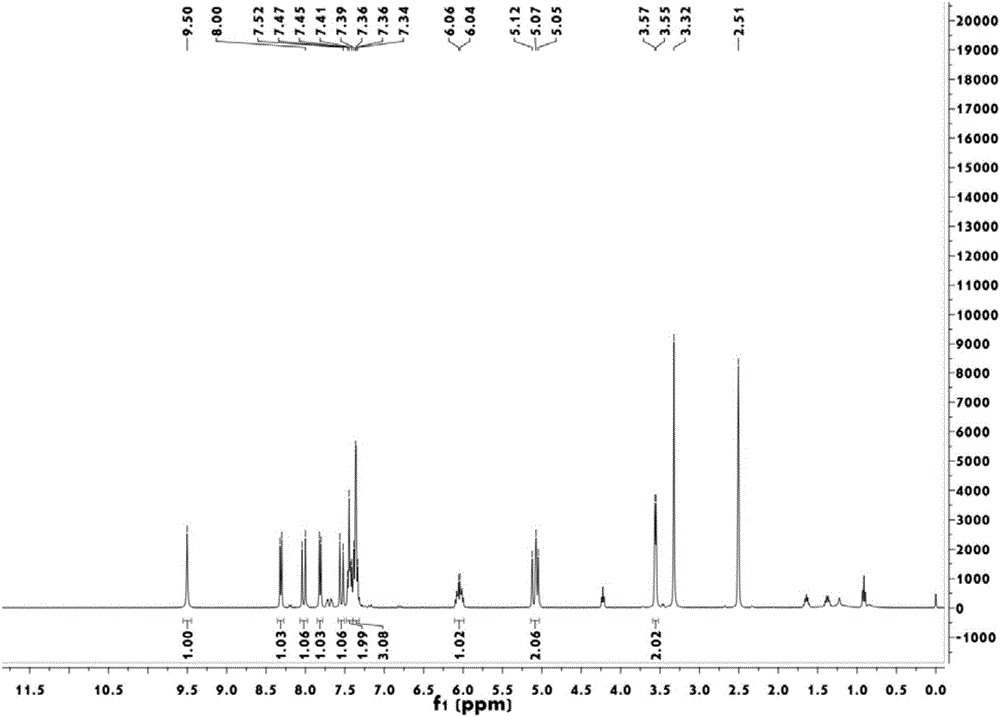
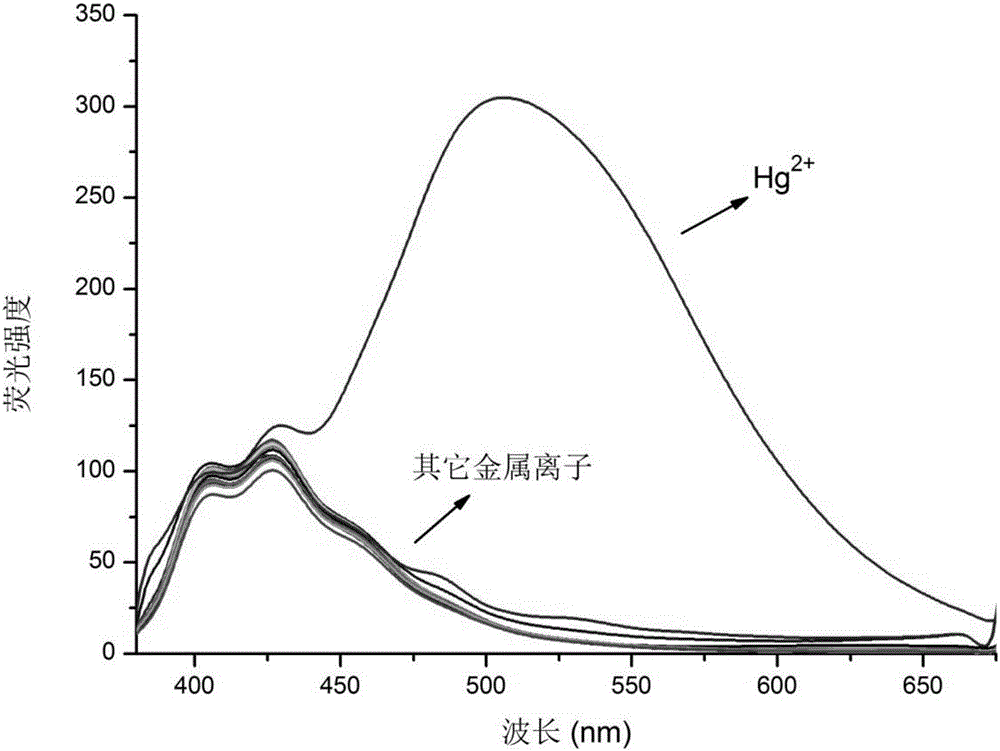
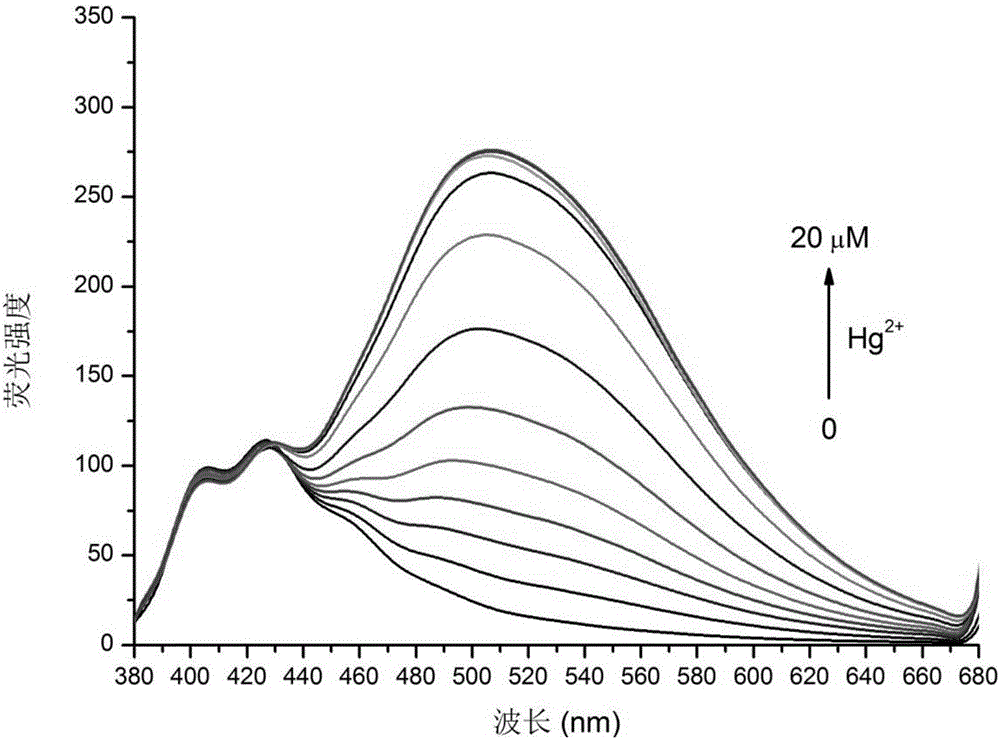
8-hydroxyquinoline mercury ion rate type fluorescent probe and preparation method thereof
A technology of hydroxyquinoline and hydroxyquinaldine, which is applied in the field of metal ion probes, can solve the problems of long response time and low detection sensitivity, and achieve the effect of strong specificity and reliable and stable detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The synthetic route of the preparation method provided by the present invention is as follows:
[0033]
Embodiment 1
[0035] This embodiment is to prepare C according to the above-mentioned preparation method 20 h 15 Specific examples of ClFNO.
[0036] 9.43mmol of 8-hydroxyquinaldine and 11.32mmol of 2-chloro-6-fluorobenzaldehyde were dissolved in 10ml of acetic anhydride and mixed, heated and stirred at 140°C, condensed and refluxed for 18 hours, and cooled to room temperature. Purified by column chromatography, the eluent was petroleum ether / ethyl acetate, and a yellow-brown solid was isolated, which was the first product 1.
[0037] Dissolve 6.2 mmol of the first product 1 and 10 ml of pyridine, heat and stir at 130° C., add 5 ml of water, stir and heat, continue to condense and reflux for 4 hours, and cool to room temperature. Purified by column chromatography, the eluent was petroleum ether / ethyl acetate, and an off-white solid was obtained, which was the second product 1.
[0038] Mix 5.1 mmol of the second product 1 with 30 ml of acetone, 1 ml of 3-bromopropene and 8 mmol of K 2 C...
Embodiment 2
[0042] This embodiment is to prepare C according to the above-mentioned preparation method 20 h 15 Specific examples of ClFNO.
[0043] 9.43mmol of 8-hydroxyquinaldine and 11.32mmol of 2-chloro-6-fluorobenzaldehyde were dissolved in 10ml of acetic anhydride and mixed, heated and stirred at 145°C, condensed and refluxed for 20 hours, and cooled to room temperature. Purified by column chromatography, the eluent was petroleum ether / ethyl acetate, and a yellow-brown solid was isolated, which was the first product 2.
[0044] Dissolve 6.2 mmol of the first product 2 and 10 ml of pyridine, heat and stir at 135° C., add 10 ml of water, stir and heat, condense and reflux for 4 hours, and cool to room temperature. Purified by column chromatography, the eluent was petroleum ether / ethyl acetate, and an off-white solid was obtained, which was the second product 2.
[0045] Mix 5.1 mmol of the second product 2 with 30 ml of acetone, 1 ml of 3-bromopropene and 8 mmol of K 2 CO 3Mixed, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com