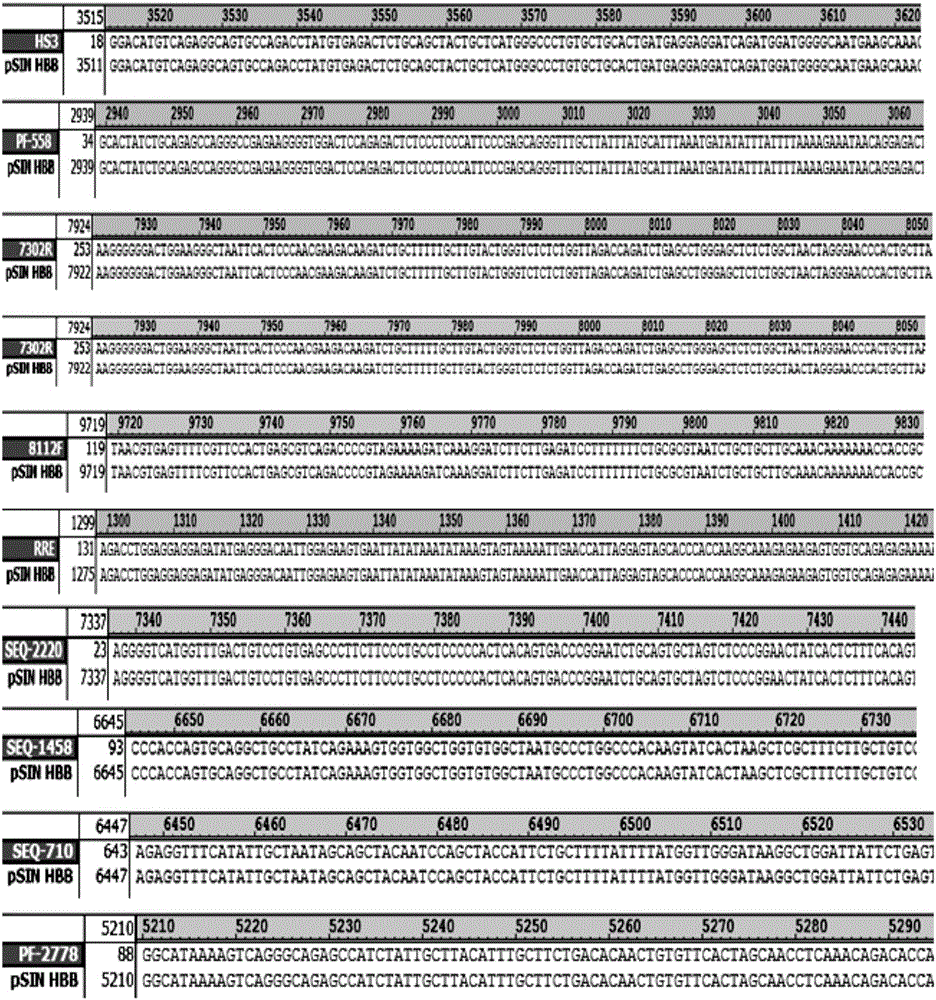
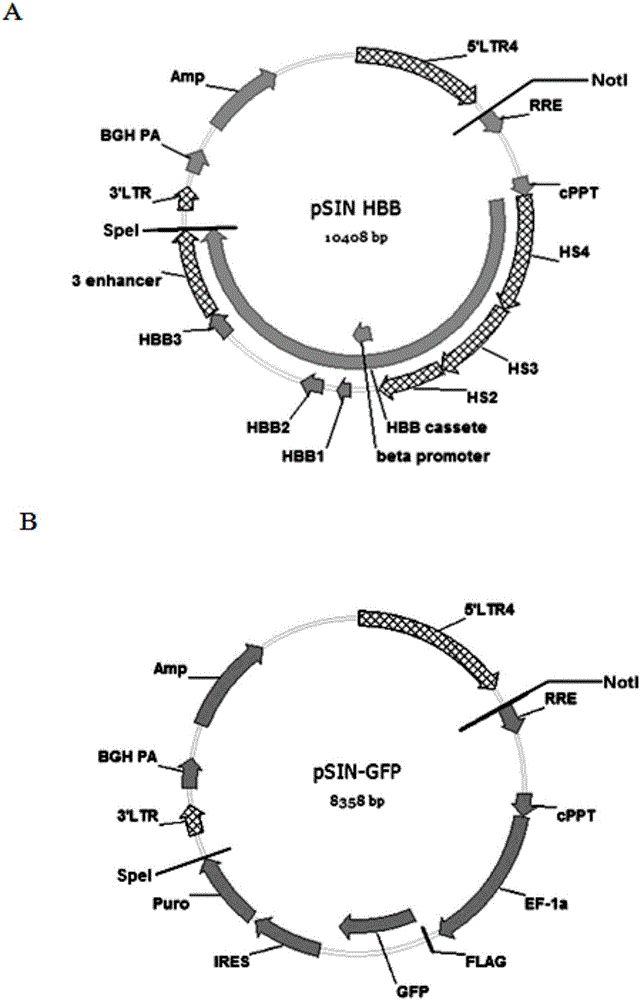
HBB gene kit for correcting autologous hematopoietic stem cell of patient suffering from servious beta-thalassemia
A technology of hematopoietic stem cells and thalassemia, which is applied in blood/immune system cells, animal cells, gene therapy, etc., to achieve the effects of broad development prospects, high virus titers, and simple application and operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Acquisition of specific HBB gene sequence
[0042] According to the human HBB gene sequence retrieved by NCBI, the retrieval sequence number is NC_000011, and specific primers were designed to obtain the full length of the HBB gene.
[0043] 1. Collect peripheral blood from normal people, and extract total DNA using the Chelex-100 method (see the instructions of the blood DNA extraction kit).
[0044] 2. Use Primer Premier 5 software to design specific primers as follows (SEQ ID No.1&SEQ ID No.2): HBB gene full-length clone upstream primer 5'-GGATCTTCCA GAGATTGTAC GGCTGTCATC ACTTAG-3' HBB gene full-length clone downstream primer 5'- CTGCCGTTCG ACGATTAAGG AACACTTCAGGGGAAAG-3'
[0045] 3. Carry out PCR amplification according to the reaction system in Table 1.
[0046] Table 1
[0047]
[0048] 4. Bio-Rad C1000 PCR instrument (Bio-Rad, USA) was used for PCR reaction. The reaction system was pre-denaturation at 98°C for 3min; Extend for 2 min, and end ...
Embodiment 2
[0052] Example 2: Construction of pSIN HBB-101 HIV lentiviral expression plasmid
[0053] 1. Use SpeI and NotI to digest the HBB-101 gene fragment, and digest at 37°C for 2 hours. The enzyme digestion reaction system is as follows:
[0054]
[0055] 2. Recover the pSIN HIV lentiviral expression plasmid by double digestion with SpeI and NotI and make it linear. Carry out the enzyme digestion reaction at 37°C for 2 hours according to the following table. The enzyme digestion reaction system is as follows:
[0056]
[0057] 3. Cloning Preparation
[0058] (1) connection
[0059] The reaction system is:
[0060]
[0061]
[0062] (2) conversion
[0063] ① Transfer 200 μl with a cooled sterile pipette tip to a sterile centrifuge tube, add 2 μl of connection solution, mix well, and place on ice for 30 minutes.
[0064] ②Heat in a constant temperature water bath at 42°C for 90 seconds, and then quickly place it on ice for 2 minutes.
[0065] ③ Add the ligated transfo...
Embodiment 3
[0074] Example 3: Construction of pSIN GFP HIV lentiviral expression plasmid
[0075] 1. Obtain the GFP gene sequence retrieved according to NCBI, the retrieval sequence number is NC_011521.1, and design specific primers to obtain the full length of the GFP gene.
[0076] 2. Use Primer Premier 5 software to design GFP gene-specific primers as follows (SEQ ID No.4&SEQ ID No.5):
[0077] GFP gene upstream primer: 5'-GGACTAGTTGAGTAAAGG-3'
[0078] GFP gene downstream primer: 5'-TTGCGGCCGCTTATTTGTA-3'
[0079] 3. Using the plasmid pEGFP-N3 as a template, the full-length GFP gene was cloned, and the GFP gene fragment was recovered by agarose gel electrophoresis.
[0080] 4. Use SpeI and NotI to digest the GFP gene fragment, and digest at 37°C for 2 hours. The enzyme digestion reaction system is as follows:
[0081]
[0082]
[0083] 5. Recover the pSIN HIV lentiviral expression plasmid by double digestion with SpeI and NotI and make it linear. Carry out the enzyme digestio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com