High-stability famciclovir tablet and preparation method thereof
A technology of famciclovir and high stability, which is applied in the field of high stability famciclovir tablets and its preparation, can solve the problems of intolerance to storage and high impurities of famciclovir tablets, and achieve the effect of high dissolution rate and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
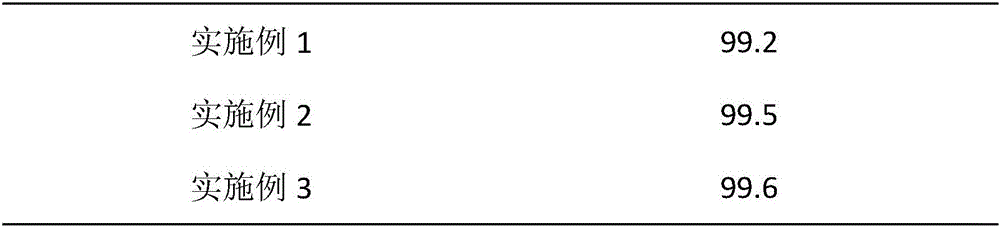
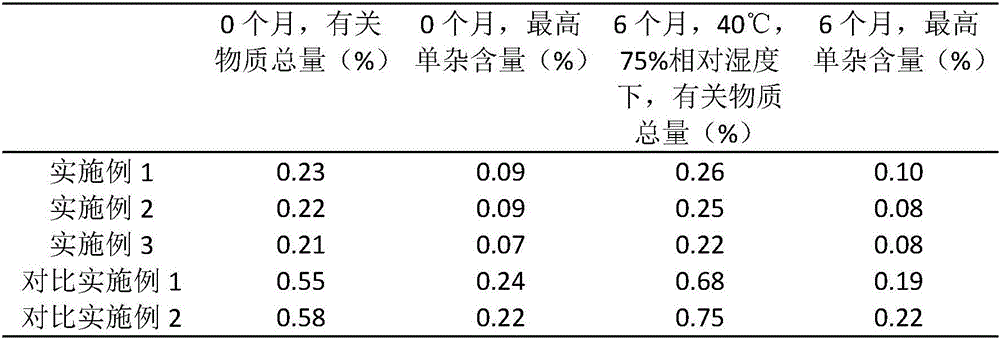
Embodiment 1
[0021] (1) Famciclovir, mannitol, carboxymethyl starch sodium and sodium bicarbonate are mixed, and the weight ratio of described famciclovir, mannitol, carboxymethyl starch sodium and sodium bicarbonate is 3:1:0.5:0.8;
[0022] (2) Add carmellose ethanol solution of 3.5% by weight to the resultant of step (1) to make soft material, granulate through a 14 mesh sieve, dry at 65-75° C., and then sieve through a 20 mesh sieve grain;
[0023] (3) add magnesium stearate and crospovidone to step (2) gained, the add-on of magnesium stearate is 5% of famciclovir weight, the add-on of crospovidone is 3% of famciclovir weight , carry out tabletting, that is to say.
Embodiment 2
[0025] (1) Famciclovir, mannitol, carboxymethyl starch sodium and sodium bicarbonate are mixed, and the weight ratio of described famciclovir, mannitol, carboxymethyl starch sodium and sodium bicarbonate is 5:1.5:0.6:1;
[0026] (2) Add carmellose ethanol solution of 2.8% by weight to the result of step (1) to make soft material, pass through 14 mesh sieve to granulate, dry at 65-75°C, and then sieve through 20 mesh grain;
[0027] (3) add magnesium stearate and crospovidone to step (2) gained, the add-on of magnesium stearate is 8% of famciclovir weight, the add-on of crospovidone is 5% of famciclovir weight , carry out tabletting, that is to say.
Embodiment 3
[0029] (1) Famciclovir, mannitol, carboxymethyl starch sodium and sodium bicarbonate are mixed, and the weight ratio of described famciclovir, mannitol, carboxymethyl starch sodium and sodium bicarbonate is 4.6:1.3:0.55:0.85;
[0030] (2) Add carmellose ethanol solution of 3% by weight to the result of step (1) to make a soft material, pass through a 14 mesh sieve to granulate, dry at 65-75°C, and then sieve through a 20 mesh sieve grain;
[0031] (3) add magnesium stearate and crospovidone to step (2) gained, the add-on of magnesium stearate is 6% of famciclovir weight, the add-on of crospovidone is 4% of famciclovir weight , carry out tabletting, that is to say.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com