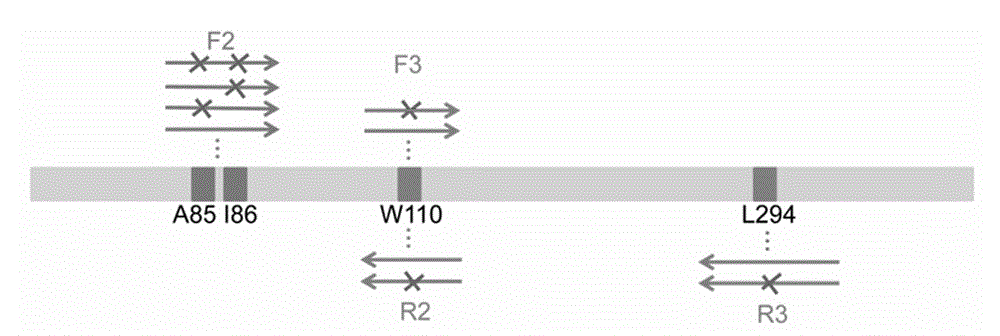
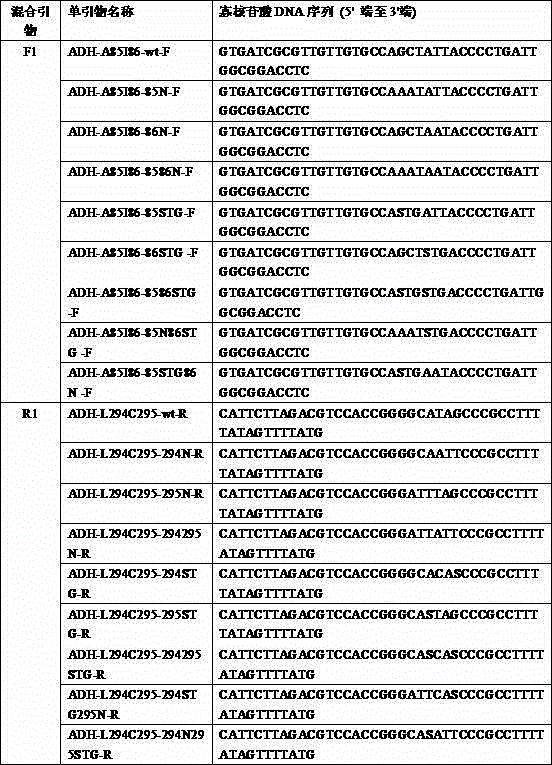
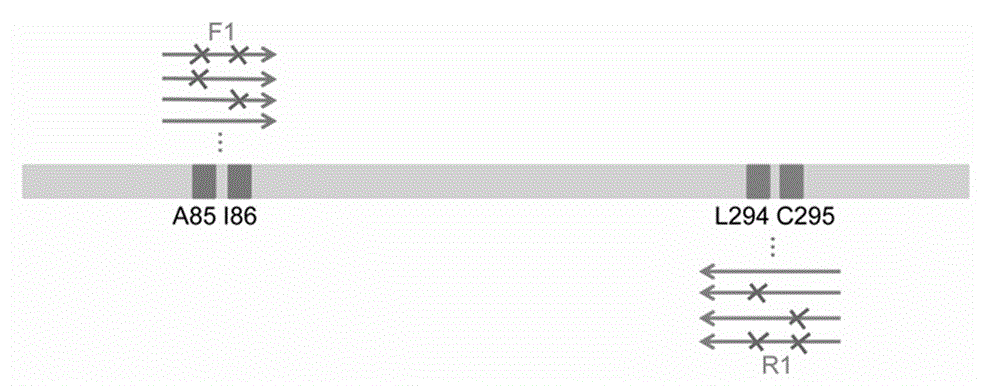Process for enzymatic preparation of chiral 3-hydroxytetrahydrofuran and alcohol dehydrogenase mutant
A technology of hydroxytetrahydrofuran and ketotetrahydrofuran, which is applied in the field of enzymatic preparation of chiral 3-hydroxytetrahydrofuran and alcohol dehydrogenase mutants, can solve the problem of high conversion rate and achieve low environmental pollution, high purity and conversion rate and efficiency high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] In the reaction system, the pH value is 7.0, the concentration is 50 mmol / L, phosphate buffer, 5mmol / L 3-ketotetrahydrofuran as the substrate and the final concentration is 10% isopropanol, 0.01mmol / L NAD + React with the crude enzyme solution of alcohol dehydrogenase mutant A85V / I86N / L294V / C295N at 30°C for 14-16 hours, extract the product and substrate with ethyl acetate and detect the reaction process with GC, measure the ee value and Conversion rate; the product is (R )-3-Hydroxytetrahydrofuran, the ee value is 97%, and the conversion rate reaches 97%.
Embodiment 2
[0065] In the reaction system, the pH value is 7.4, and the concentration is 100 mmol / L phosphate buffer solution, 5 mmol / L 3-ketotetrahydrofuran as the substrate and the final concentration is 10% isopropanol, 0.05 mmol / L L-NAD + As a cofactor and I86N / C295N alcohol dehydrogenase mutant crude enzyme solution, react at 25°C for 14-16 hours, extract the product and substrate with ethyl acetate and use GC to detect the reaction process, measure the ee value and conversion rate , the product is ( R )-3-hydroxytetrahydrofuran, the ee value is 99%, and the conversion rate is 99%.
Embodiment 3
[0067] In the reaction system, add phosphate buffer solution with a pH value of 7.4 and a concentration of 50mmol / L, 5mmol / L 3-ketotetrahydrofuran as a substrate and isopropanol with a final concentration of 10%, 0.01~0.1 mmol / LNADP + As a cofactor and I86V / W110L / L294Q alcohol dehydrogenase mutant lysate, react at 30°C for 14-16 hours, extract the product and substrate with ethyl acetate and use GC to detect the reaction process, determine the ee value and conversion rate; the product is ( S )-3-hydroxytetrahydrofuran, the ee value is 95%, and the conversion rate is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com