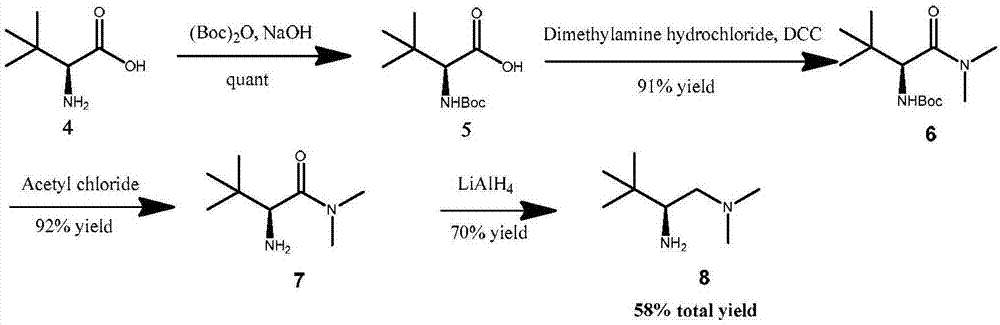
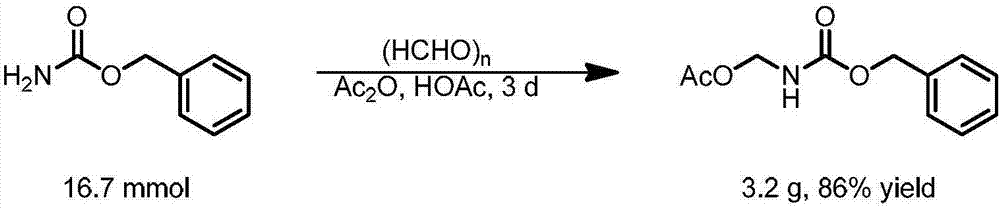
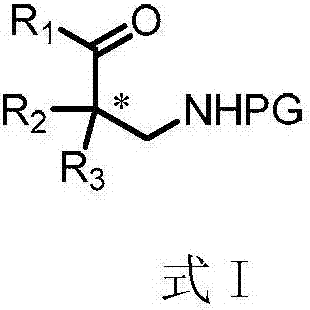
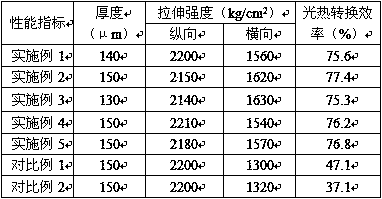
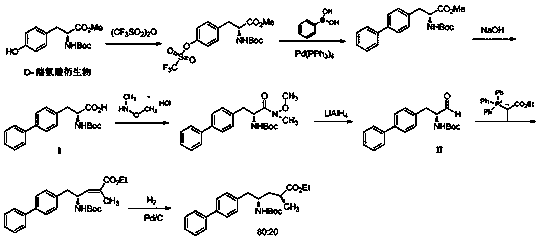
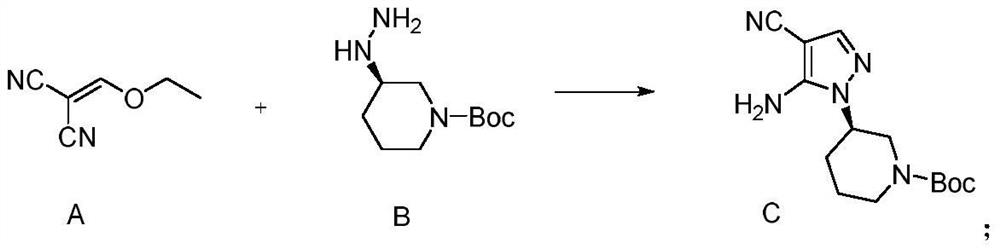
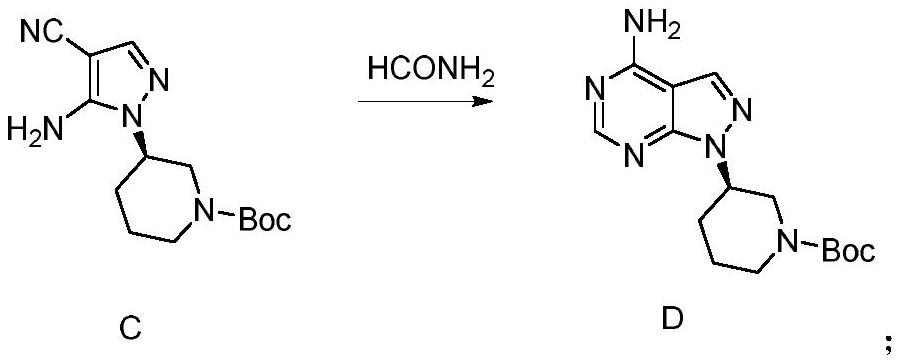
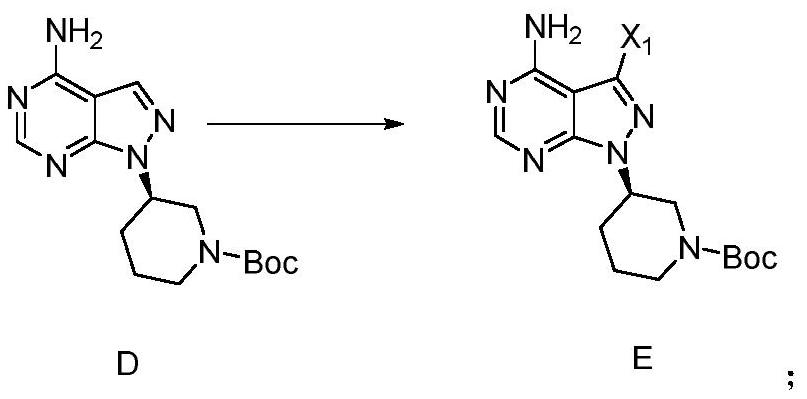
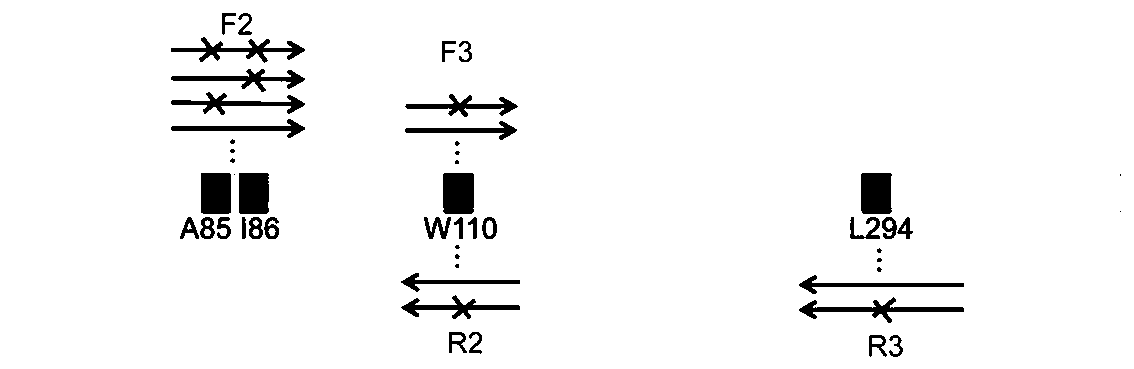
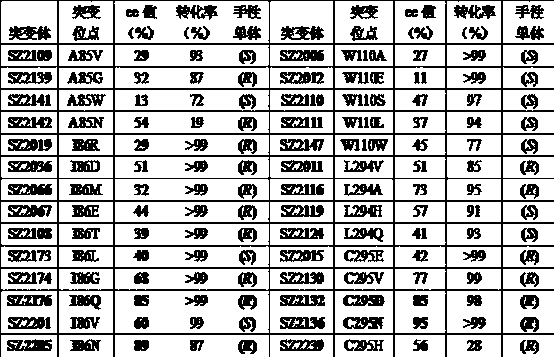
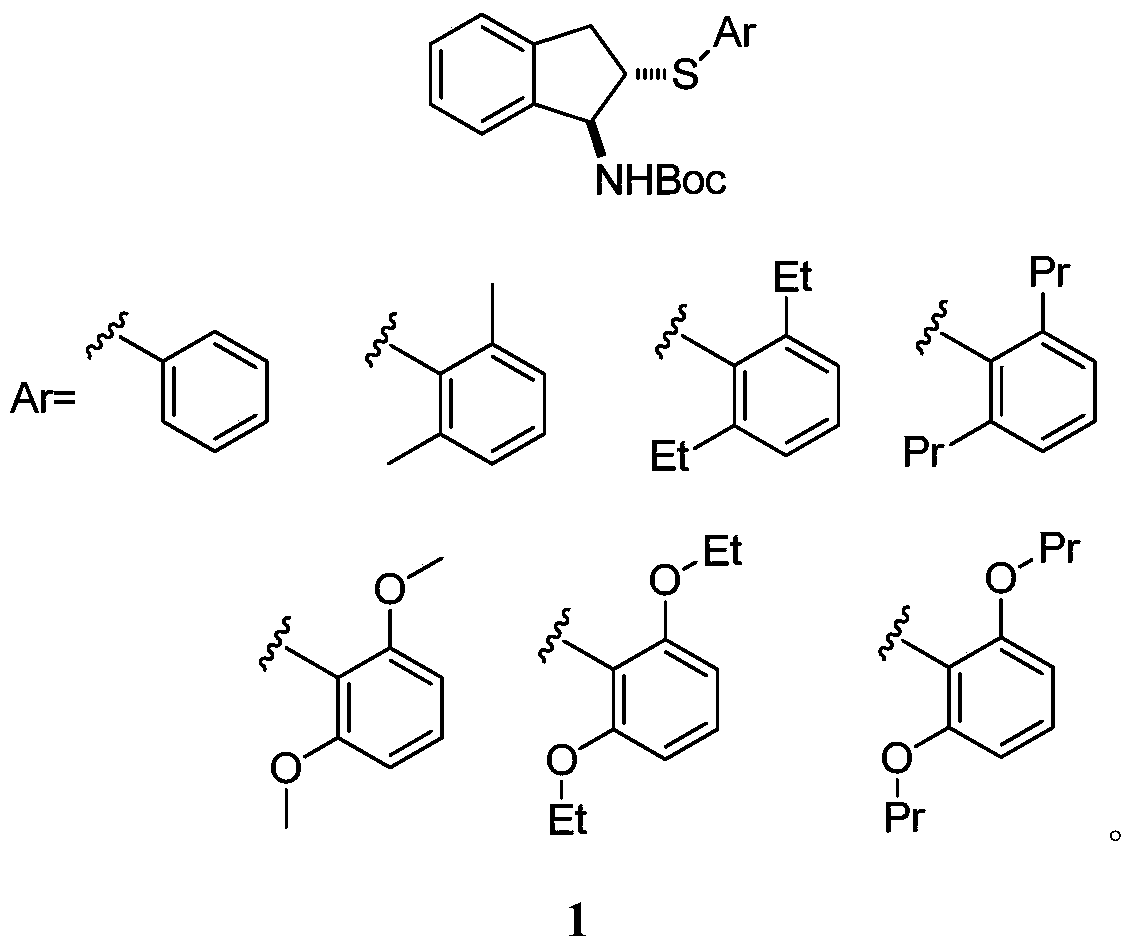
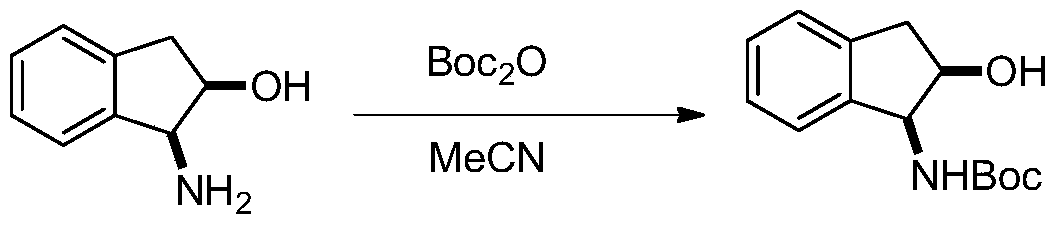Patents
Literature
52results about How to "Good optical selectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Organic EL Light Emitting Device and Manufacturing Method Thereof
InactiveUS20130334507A1Good optical selectivityReducing optical transparencySolid-state devicesSemiconductor/solid-state device manufacturingOptical transparencyLight emitting device
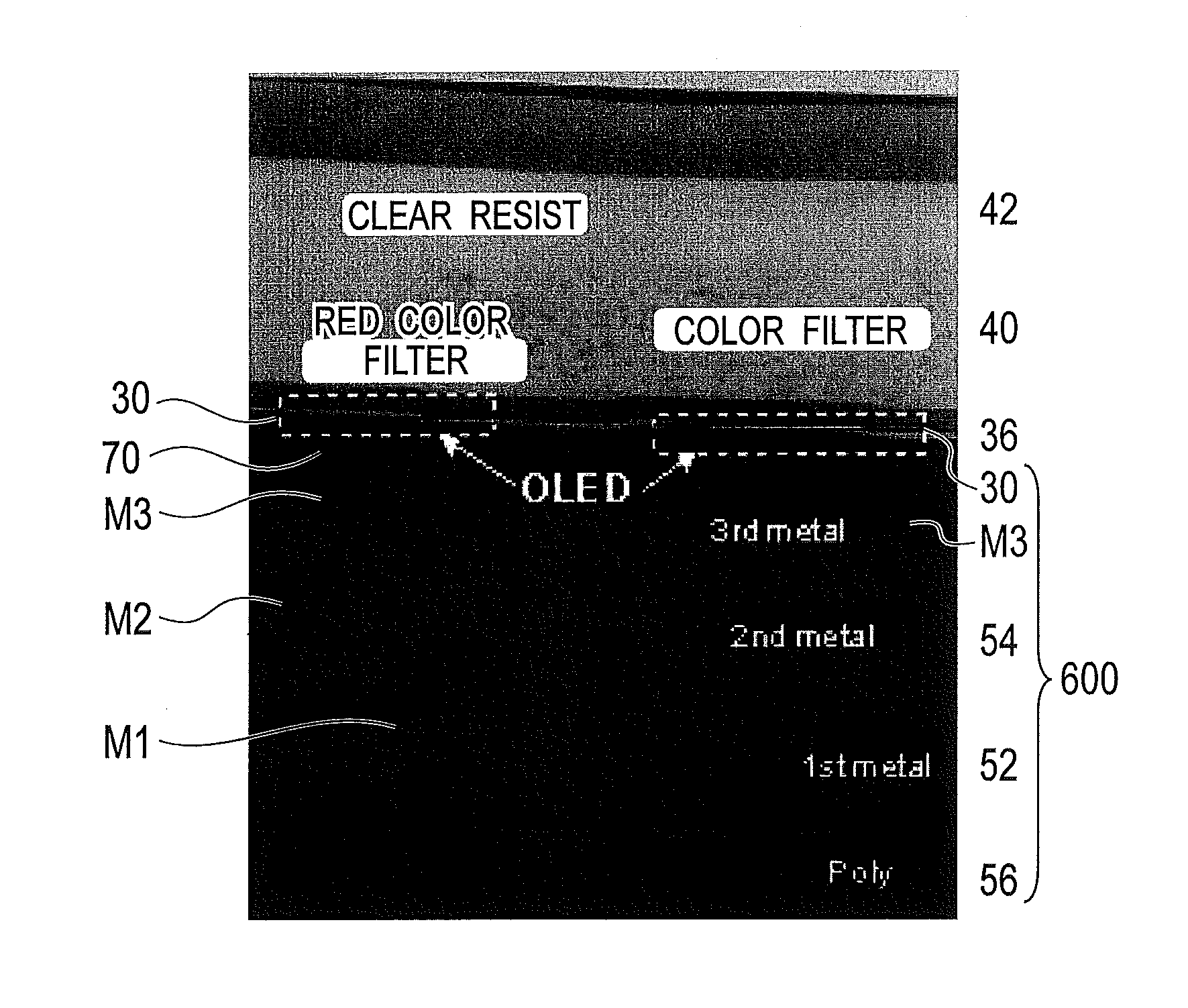
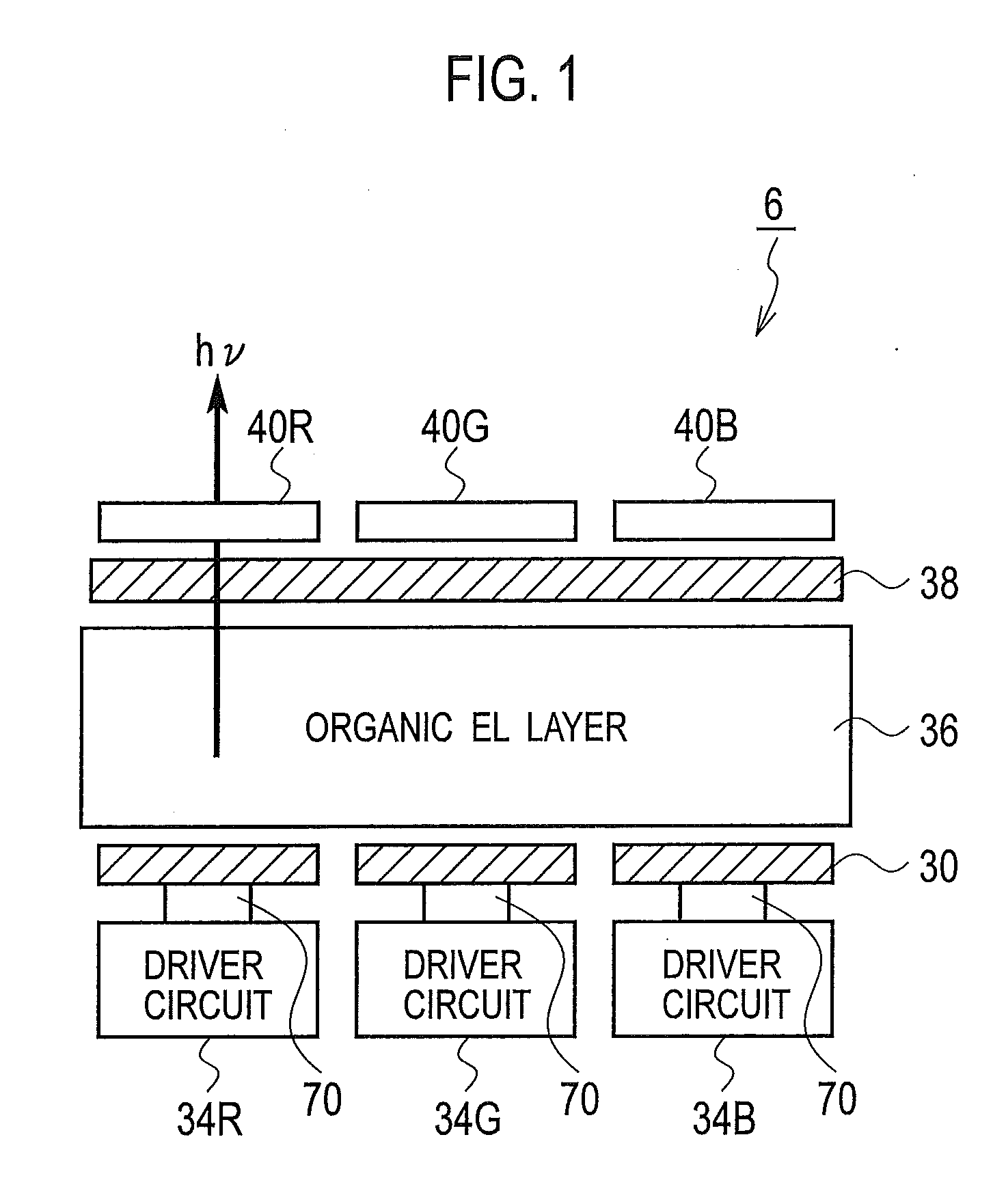
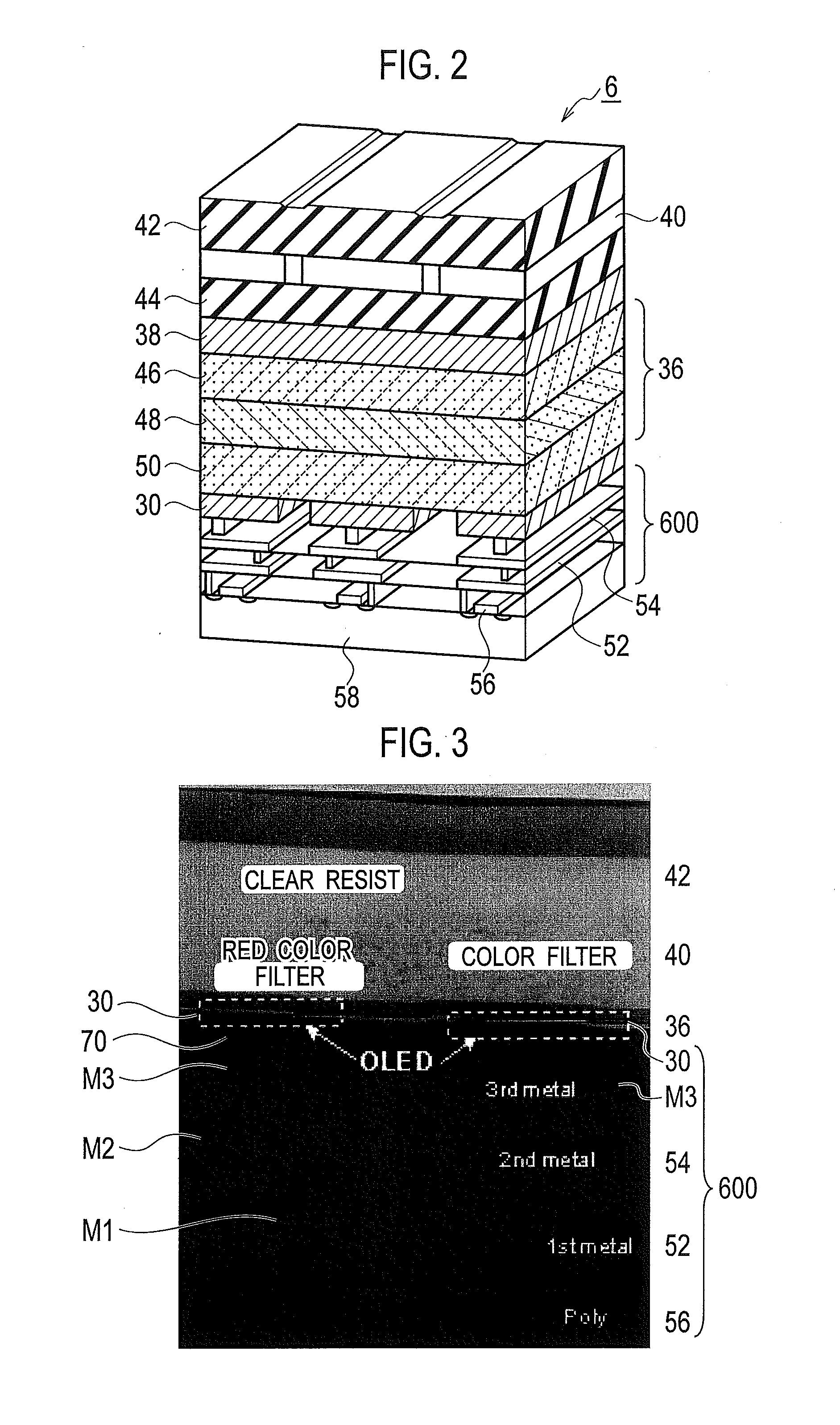
There is provided a layered color filter which can improve optical selectivity, without reducing optical transparency, an organic EL light emitting device on which such a layered color filter is mounted, and a fabrication method of such an organic EL light emitting device. The layered color filter includes a substrate 60, a red color filter 40R1, 40R2, a green color filter 40G1, 40G2, and a blue color filter 40B1, 40B2, 40B3 disposed on the substrate 60. In the layered color filter, the organic EL light emitting device on which such a layered color filter is mounted, and the fabrication method thereof, at least one color filter among the red color filter 40R1, 40R2, the green color filter 40G1, 40G2, and the blue color filter 40B1, 40B2, 40B3 is laminated to be formed as a plurality of thin film layers.
Owner:ROHM CO LTD
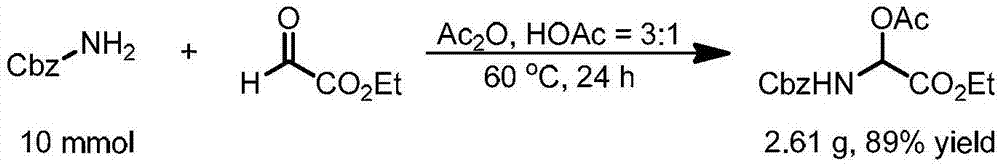
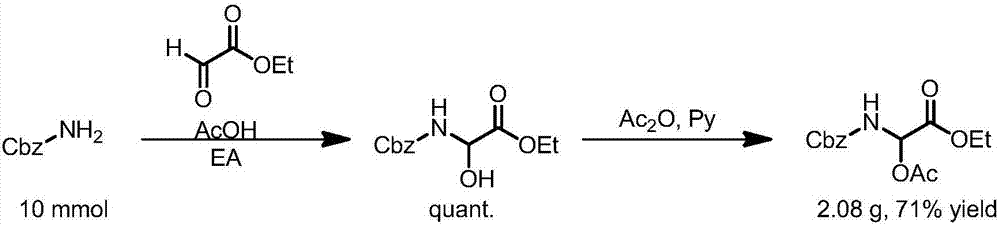
Sacubitril intermediate and preparation method of sacubitril intermediate and sacubitril
ActiveCN105924355AImprove qualityHigh yieldOrganic compound preparationCarboxylic acid esters preparationChemical reactionOrganic synthesis
The invention discloses a sacubitril intermediate and a preparation method of the sacubitril intermediate and sacubitril, and belongs to the technical field of organic synthesis of drugs. On one hand, the invention discloses a novel compound-sacubitril intermediate compound B and a preparation method thereof, and on the other hand, the invention discloses a novel preparation method of the sacubitril. The sacubitril intermediate and the preparation method of the sacubitril intermediate and the sacubitril have the following advantages that the synthesizing route is short, the product can be prepared only through four steps of chemical reactions, and the steps of existing patent routes all exceed nine steps; the dicarbonyl compound is prepared through Reformasky condensation supplied by the method, and the cost is low; a first chiral center is introduced by fully utilizing a chiral compound L-(S)-ethyl lactate which is low in cost and easy to obtain in the natural world, and the cost is low; a second chiral center is constructed through a biological enzyme catalysis technique, the optical selectivity reaches up to 99.9%, the quality is good, and the cost is low. By means of the technological means, the total yield of the prepared sacubitril is increased, and the quality of the sacubitril is improved.
Owner:ZHEJIANG HONGYUAN PHARMA
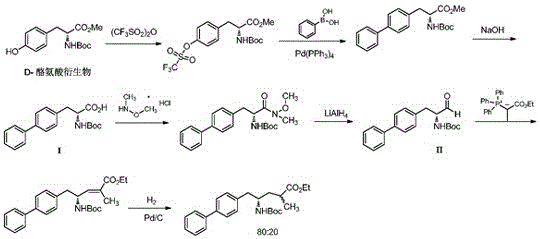
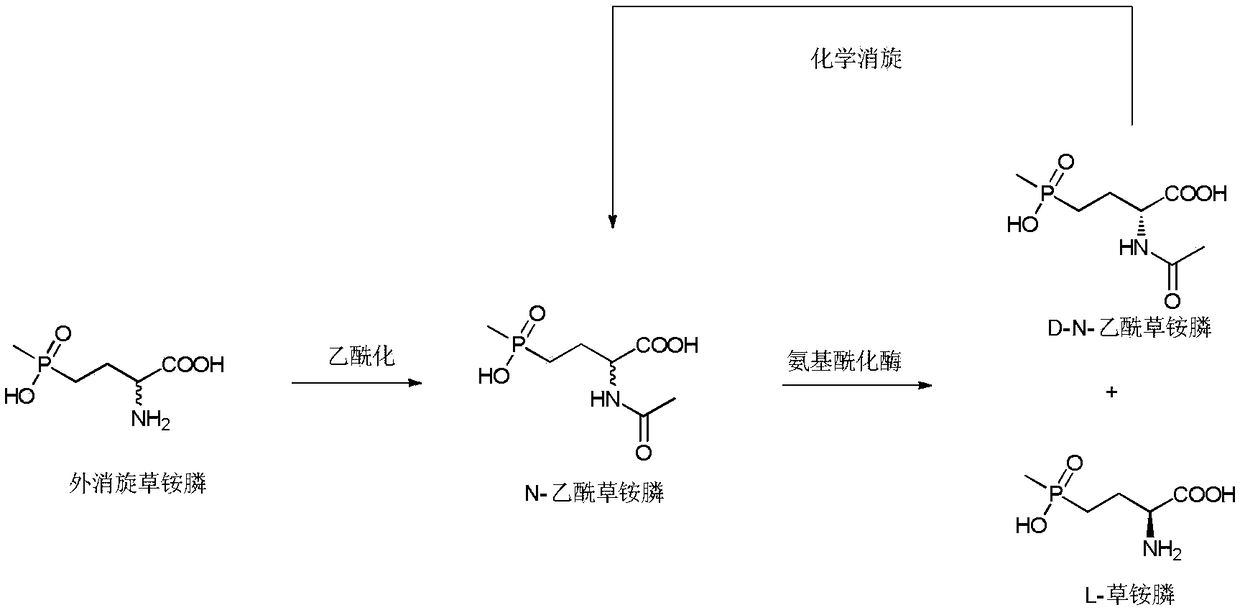
Method of utilizing chemistry-enzyme method to produce L-glufosinate ammonium
The invention discloses a method of utilizing a chemistry-enzyme method to produce L-glufosinate ammonium. According to the method, racemic N-acetyl glufosinate ammonium is taken as the substrate, engineering bacteria containing carboxyl-peptidase genes are subjected to fermentation culture to obtain wet cells, wet cells or pure enzymes, which are obtained by steps of grinding wet cells through ultrasonic waves and extracting grinded wet cells, are taken as the catalyst, a buffer solution with a pH value of 5-10 is taken as the reaction medium, reactions are performed in a water bath with a temperature of 45 DEG C at a rotation speed of 200 rpm, after complete reactions, a reaction solution containing D-N-acetyl glufosinate ammonium and L-glufosinate ammonium is obtained, the reaction solution is separated and purified, and collected D-N-acetyl glufosinate ammonium is subjected to racemization and then split in cycles to obtain L-glufosinate ammonium at the same time. The provided method does not need a coenzyme circulation system or an amino donor with a structure that is similar with the product; the reaction steps are simple, the reaction product is easy to separate, the opticalselectivity is high (ee value is 99%), the separation effect of the ion exchange column is obvious, and L-glufosinate ammonium with higher purity can be obtained more easily (purity is 98%).
Owner:ZHEJIANG UNIV OF TECH
Organic catalyst containing primary amine, tertiary amine and urea or thiourea and preparation method thereof
InactiveCN101597286ANovel structureUnique structureOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsThio-Thiourea
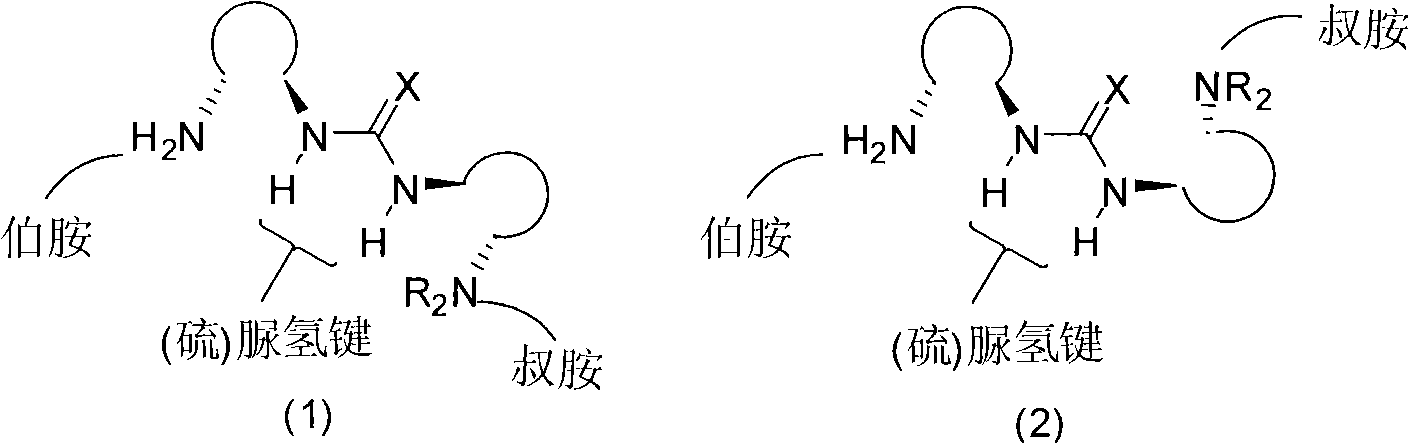
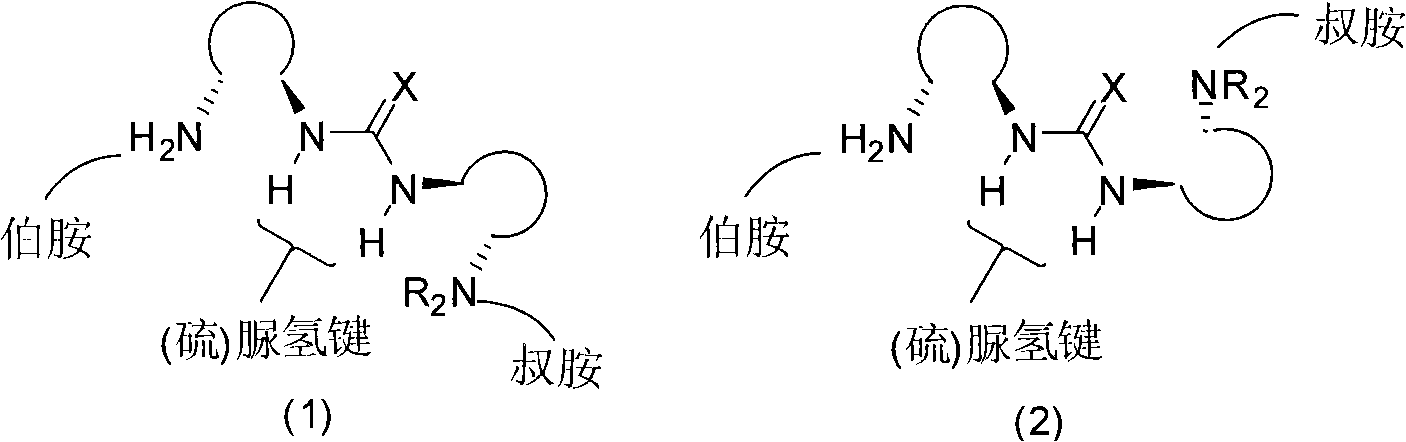
The invention relates to an organic catalyst, in particular to an organic catalyst containing primary amine, tertiary amine and urea or thiourea and a preparation method thereof. One catalyst molecule respectively includes one or more primary amine functional groups, one ore more tertiary amine functional groups and one or more urea or thiourea functional groups. The designed multi-functional organic catalyst is novel in structure and simple and convenient in preparation method; since a plurality of the functional groups of primary amine, tertiary amine and thio(urea) exist in the organic catalyst molecule, the good synergetic effect can be shown during the catalytic asymmetric organic reaction, the high catalytic efficiency and the high optical selectivity can be displayed, and the organic catalyst can be well applied to the asymmetric reaction of various types of organic catalysis and has wide application range.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of solar selective-absorption thin film with spinel structure
InactiveCN103691647ANo heat treatment requiredNo oxidation problemsPretreated surfacesRadiation-absorbing paintsLow emissivityPolyethylene glycol
The invention belongs to the field of solar heat utilization, and discloses a preparation method of a solar selective-absorption thin film with a spinel structure. The selective-absorption thin film is subjected to pulling and film plating through a sol-gel method and is formed by roasting. The preparation method is characterized in that metal salt of copper, cobalt and manganese is used as a sol precursor body, alcohol is used as a solvent, metal sol impregnation liquid is prepared by mixing the sol precursor body and the solvent with hydroxyl carboxylic acid and polyethylene glycol 200 according to a certain ratio, a layer of thin film can be plated on a metal substrate having an infrared reflection characteristic by adopting a pulling method after a film forming agent is added, an obtained thin film has higher solar absorption rate (0.90-0.93) and lower emissivity (0.05-0.07) after being subjected to 80-100 DEG C solidification for 30-60 minutes and then being subjected to 500 DEG C heat treatment for 30-60 minutes, and the photo-thermal conversion efficiency of a solar heat collector can be effectively increased.
Owner:CHANGZHOU UNIV
Process for enzymatic preparation of chiral 3-hydroxytetrahydrofuran and alcohol dehydrogenase mutant
ActiveCN106520714AReduce pollutionImprove efficiencyMicroorganism based processesOxidoreductasesAlcohol3-Hydroxytetrahydrofuran
The invention relates to a process for enzymatic preparation of chiral 3-hydroxytetrahydrofuran and an alcohol dehydrogenase mutant. The process comprises the a step of, with 3-ketotetrahydrofuran as a substrate, subjecting the substrate, the alcohol dehydrogenase mutant, a cofactor and a cofactor regeneration system to a biological catalysis reaction in a buffer system so as to produce chiral 3-hydroxytetrahydrofuran, wherein for alcohol dehydrogenase used by the alcohol dehydrogenase mutant, the wild gene sequence of alcohol dehydrogenase is derived from thermophilic bacteria, the base sequence is as shown in SEQ ID No. 1, and the amino acid sequence is as shown in SEQ ID No. 2. Compared with chemical methods, the process using the alcohol dehydrogenase mutant for preparation of chiral 3-hydroxytetrahydrofuran has the advantages of small environmental pollution, high efficiency, high optical selectivity; and compared with enzyme resolution methods, the process provided by the invention has higher purity and conversion rate and has ee value and conversion rate of both 99%.
Owner:洛阳华荣生物技术有限公司
Method for preparing chiral 2-chloro-3,4-difluorophenethyl alcohol
ActiveCN106520849AHigh product yieldGood optical selectivityChemical recyclingFermentationChemistryAmino acid
The invention discloses a method for preparing chiral 2-chloro-3,4-difluorophenethyl alcohol. According to the method, 2-chloro-3,4-difluoroacetophenone is used as a substrate, and ketoreductase is used for catalytic reduction of the substrate so as to generate the chiral 2-chloro-3,4-difluorophenethyl alcohol. Amino acid sequence of the ketoreductase is as shown in the SEQ ID NO.1-21. 2-chloro-3,4-difluoroacetophenone which is cheap and easily available is used as the substrate, and the biocatalyst ketoreductase is adopted so as to carry out an asymmetric reduction reaction to obtain 2-chloro-3,4-difluorophenethyl alcohol with high chiral purity. The method has advantages of high yield, mild reaction condition and simple operation. Problems of a chemical reduction method, such as strict reaction condition, complex preparation of a catalyst, high cost, inflammable property and low chiral purity of the product, are avoided. The method has good practical industrial application value.
Owner:杭州酶易生物技术有限公司
Thermal spraying coating layer antireflection layer suitable for solar selective absorption and preparation method thereof
InactiveCN105239060AAlleviate excessive stressAvoid crackingLiquid/solution decomposition chemical coatingPorosityThermal spraying
The invention belongs to the field of coating layer antireflection materials, and in particular, relates to a thermal spraying coating layer antireflection layer suitable for solar selective absorption and a preparation method thereof. The thermal spraying coating layer antireflection layer consists of a single CuMnOx layer, or consists of composite two layers: an inner layer of a CuMnOx layer and an outer layer of a SnO2 layer. The CuMnOx layer is prepared by CuMnOx composite sol; and the CuMnOx composite sol is prepared by mixing nanometer solid particles in an amorphous state with CuMnOx sol. The thermal spraying coating layer antireflection layer, prepared by the method, has excellent optical selection performance, can relieve the stress effect of a coating layer in the heat treatment process, decreases the porosity of the thermal spraying coating layer, improves the absorption emitting ratio of the coating layer, is better in heat stability under high temperature, achieves multiple effects of hole sealing, protection and reflection reduction, and is compact in structure and stable in performance.
Owner:WUHAN UNIV OF TECH
Method for catalytically synthesizing chiral curcumin analogs
ActiveCN102115446ANovel structureUnique structureOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystThiourea
The invention relates to an asymmetric chemical reaction process of catalytic conjugate addition, in particular to a method for catalytically synthesizing chiral curcumin analogs. The method comprises the steps of: taking nitroolefin and curcumin analogs as raw materials; taking tertiary amine-thiourea organic catalyst as a catalyst system; reacting in dissolvent, wherein the reaction time is 0.5-15 days, and the reaction temperature is -40-40 DEG C; and generating a conjugate addition product. The reaction general formula is shown in the description: in the formula, R1 and R2 are aliphatic series group and aromatic series group. The structural formula of the tertiary amine-thiourea organic catalyst organic catalyst is shown in the description: in the formula, R1 is tertiary amine-containing quindenary derivative, R2 and R3 are different or same aromatic series substituent groups respectively, and R4 is sulfonyl substituent group. The tertiary amine-thiourea organic catalyst organic catalyst is high in catalytic activity and stereoselectivity in the Michael addition reaction between the nitroolefin and the curcumin analogs, wherein the enantioselectivity is highest to 97%, the yield is highest to 96%, and the reaction substrate is wide in range.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing (2S, 3R)-2-benzyloxy-3-pentanol as intermediate of posaconazole
InactiveCN103936564AWide variety of sourcesLow priceOrganic compound preparationCarboxylic acid esters preparationPtru catalystBenzyl chloride
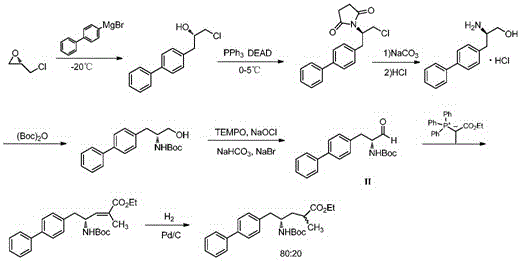
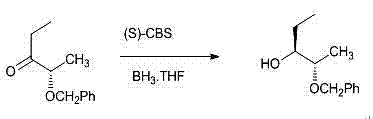
The invention relates to a method for preparing (2S, 3R)-2-benzyloxy-3-pentanol as an intermediate of posaconazole, The method comprises the following steps of directly protecting hydroxyl group of S-ethyl lactate with the benzyl chloride, carrying out a Grignard reaction to obtain (2S)-2-benzyloxy-3-pentanone and directly carrying out a chiral reduction with BH3 to obtain (2S, 3R)-2-benzyloxy-3-pentanol in the presence of a chiral catalyst, wherein the S-ethyl lactate, which is wide in source and low in cost, is used as a starting material. The method disclosed by the invention has the advantages of reduction in reaction steps, improved yield, effectively saved cost, low cost of raw materials, environmental protection, less pollution, wide source of S-ethyl lactate as the starting material, low price, relatively mild reaction conditions, high yield and greatly reduced cost. The products prepared by the method has high optical selectivity, ee values greater than 99%, purity greater than 99% and less impurities and can be used directly without purification.
Owner:JINAN KANGHE MEDICAL TECH
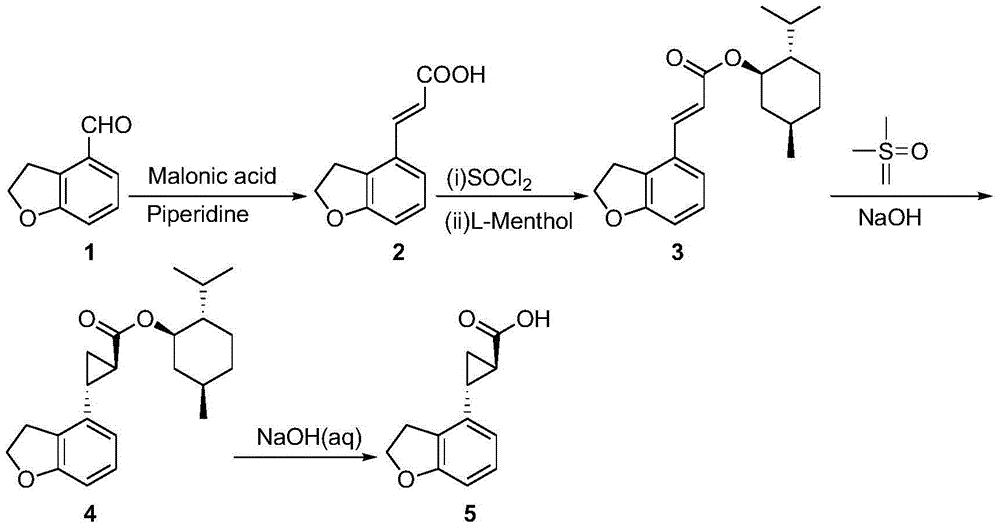
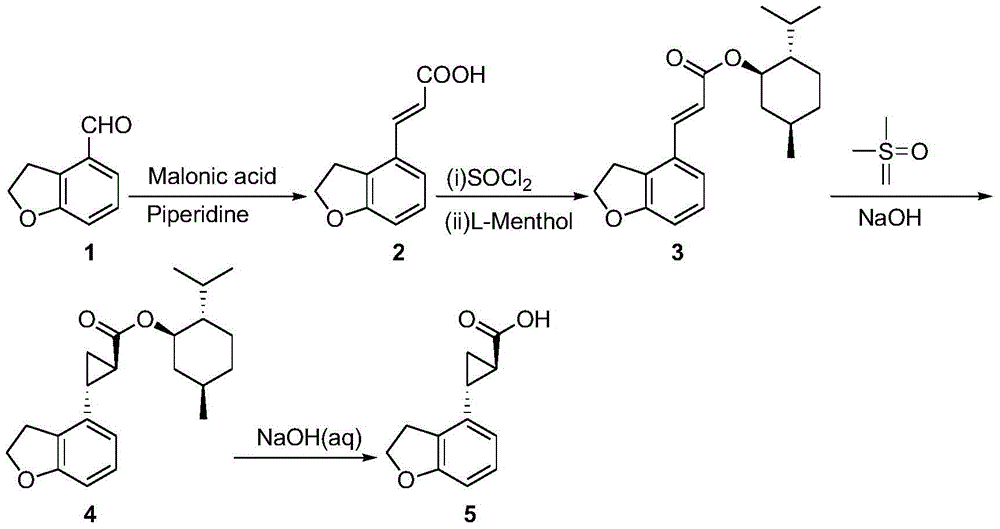
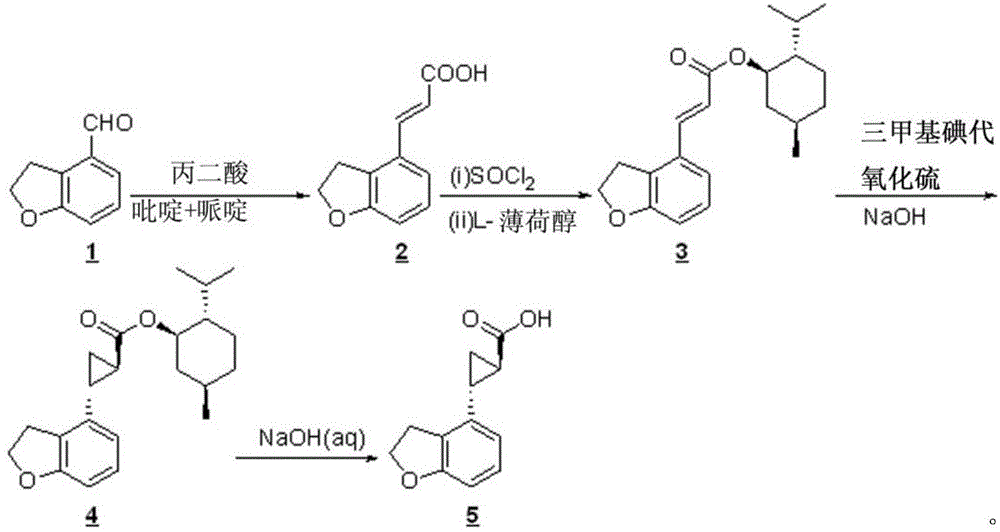
Novel preparation technology of tasimelteon intermediate
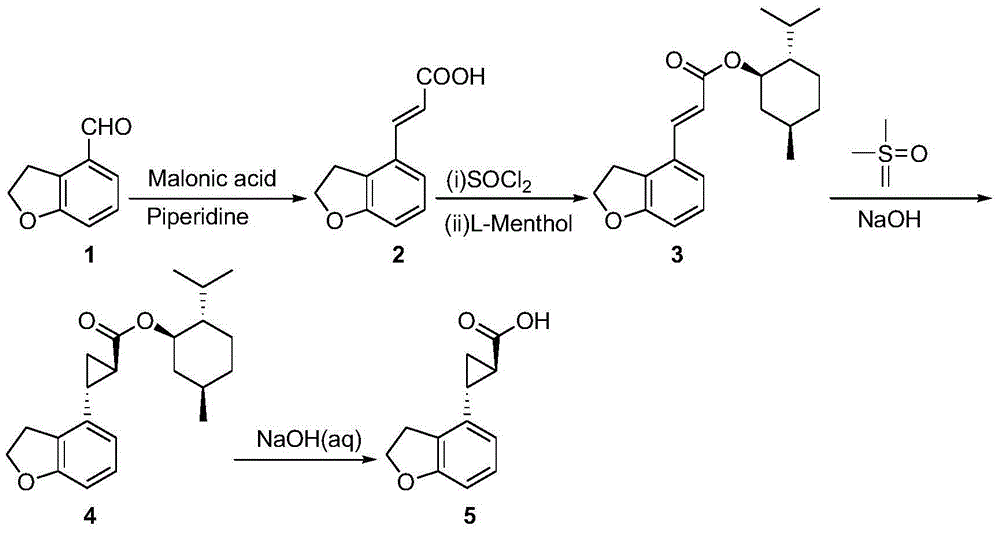
The invention provides a novel preparation technology of a tasimelteon intermediate namely (1R,2R)-(2,3-dihydrobenzofuran-4-yl)cyclopropyl formic acid. The technology comprises the following steps: (1) carrying out reactions between 2,3-dihydrobenzofuran-4-formaldehyde and malonic acid in the presence of piperidine to generate 2,3-dihydrobenzofuran-4-acrylic acid; (2) converting 2,3-dihydrobenzofuran-4-acrylic acid into acyl chloride in the presence of thionyl chloride, then reacting the acyl chloride with L-menthol to generate 2,3-dihydrobenzofuran-4-acrylic acid L-menthol; (3) carrying out reactions between 2,3-dihydrobenzofuran-4-acrylic acid L-menthol and dimethyl sulfur oxide in the presence of sodium hydroxide to generate (1R,2R)-(2,3-dihydrobenzofuran-4-yl)cyclopropyl formic acid L-menthol; (4) hydrolyzing the (1R,2R)-(2,3-dihydrobenzofuran-4-yl)cyclopropyl formic acid L-menthol in the action of a sodium hydroxide water solution so as to obtain the (1R,2R)-(2,3-dihydrobenzofuran-4-yl)cyclopropyl formic acid. The novel preparation technology is developed on the basis of the conventional technologies, and has the advantages of easily-available raw materials, low cost, simple operation, and suitability for industrial massive production.
Owner:苏州莱克施德药业有限公司
ZnO quantum dot-based deep UV sensor and preparation method thereof
ActiveCN103400900ASimple production equipmentSimple technical processFinal product manufactureSemiconductor devicesSchottky barrierDesorption
The invention relates to a ZnO quantum dot-based deep UV sensor and a preparation method thereof. A superfine ZnO quantum dot network structure serves as an active photoelectric response layer; the ZnO quantum dot-based deep UV sensor is prepared after the preparation of ZnO quantum dots. In the prior art, the switching of photocurrent is very slow due to oxygen adsorption and desorption generating on the surface of ZnO, and an effective Schottky-barrier is difficult to be designed in a device. The novel ZnO quantum dot-based deep UV sensor is designed through a simple and low-cost self-assembling process has the advantages that the problems in the prior art are solved; the spectrum selectivity is high; the optical switch action is stable; the photocurrent responsiveness is high; the response speed is high; the photocurrent switching characteristic is remarkable, fast and stable; the photocurrent on-off ratio is larger than 103; both the photocurrent rising time and decaying time are shorter than 1s; the response speed of the UV sensor is increased.
Owner:YANGZHOU UNIV
Preparation method of esomeprazole sodium
InactiveCN111635393AHigh yieldGood optical selectivityAsymmetric synthesesEsomeprazole SodiumOmeprazole Sodium
The invention provides a preparation method of esomeprazole sodium, specifically a preparation method of optically pure esomeprazole sodium. The preparation method comprises the step of preparing esomeprazole sodium by taking 2-mercapto-5-methoxy-1H-benzimidazole and 2-chloromethyl-4-methoxy-3,5-dimethyl pyridine hydrochloride as starting raw materials. The method provided by the invention has good selectivity, can obtain almost optically pure products, and is suitable for industrial production of esomeprazole sodium.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Chiral beta-amino acid derivative and preparation method thereof
ActiveCN107382783ASimple structureShort synthetic routeCarbamic acid derivatives preparationCarboxylic acid nitrile preparationStrong acidsSolvent free
The invention discloses a chiral beta-amino acid derivative and a preparation method thereof. The structural formula of the chiral beta-amino acid derivative is represented by a formula I (shown in the description). The preparation method comprises the steps of mixing a mixture of a carbonyl compound and N,O-acetal with chiral primary-tertiary diamine organic small-molecule catalyst, strong acid and weak acid to react, so as to obtain the chiral beta-amino acid derivative, wherein the carbonyl compound includes aldehyde and / or ketone. The chiral beta-amino acid derivative is catalyzed by virtue of the chiral primary-tertiary diamine organic small-molecule catalyst with a simple structure, is synthesized through a one-step method, and is solvent-free, simple and efficient.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Method for preparing 2S,3R-2-benzyloxy-3-pentanol
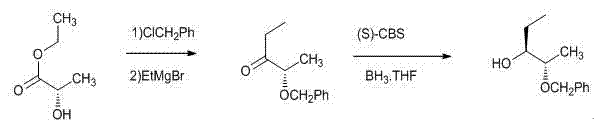
InactiveCN102795972AReduce pollutionGood optical selectivityOrganic chemistryOrganic compound preparation3-PentanolReagent
The invention discloses a method for preparing a 2S,3R-2-benzyloxy-3-pentanol intermediate for preparing posaconazole. The method comprises the following steps of: performing addition on 2S-benzyloxy-propanal and ethyl magnesium bromide at the temperature of 60 DEG C below zero to 5 DEG C under induction of a chiral aid, and performing post treatment on the reactants to obtain a crude 2S,3R-2-benzyloxy-3-pentanol product of which the enantiomeric excess (ee) value is more than 99 percent, wherein the crude product is directly used for synthesizing the posaconazole without refining. The preparation method is environment-friendly, high in optical selectivity and high in yield, and greatly reduces the cost.
Owner:成都费歇尔医药科技有限公司
Photothermal conversion plastic film for building with microporous structure, and preparation method of photothermal conversion plastic film
The invention belongs to the technical field of preparation of light conversion films, and provides a photothermal conversion plastic film for building with a microporous structure, and a preparationmethod of the photothermal conversion plastic film. According to the preparation method, a film is made by adding a light-transmitting agent and aerogel into polyvinyl alcohol resin, and the film having a photothermal conversion effect is prepared by uniaxially stretching. Since the light-transforming agent and polyvinyl alcohol are incompatible systems and the stress orientations are different ina stretching process, the film, which is stretched under the assistance of the aerogel, forms the microporous structure around the light-converting agent, the light is reflected and refracted for a plurality of times in micropores of the film, and the photothermal conversion efficiency is further increased. Compared with the traditional method, the photothermal conversion plastic film prepared bythe method is high in photothermal conversion efficiency and has higher optical selectivity and tensile property; furthermore, the whole preparation process is simple, lower in cost and little in pollution to environment, thus being widely popularized and applied.
Owner:CHENDU NEW KELI CHEM SCI CO LTD
Multiphase unsymmetrical hydrogenated catalyst, preparation method and application thereof
InactiveCN101284230AHigh activityGood optical selectivityOrganic reductionOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen pressureSolvent
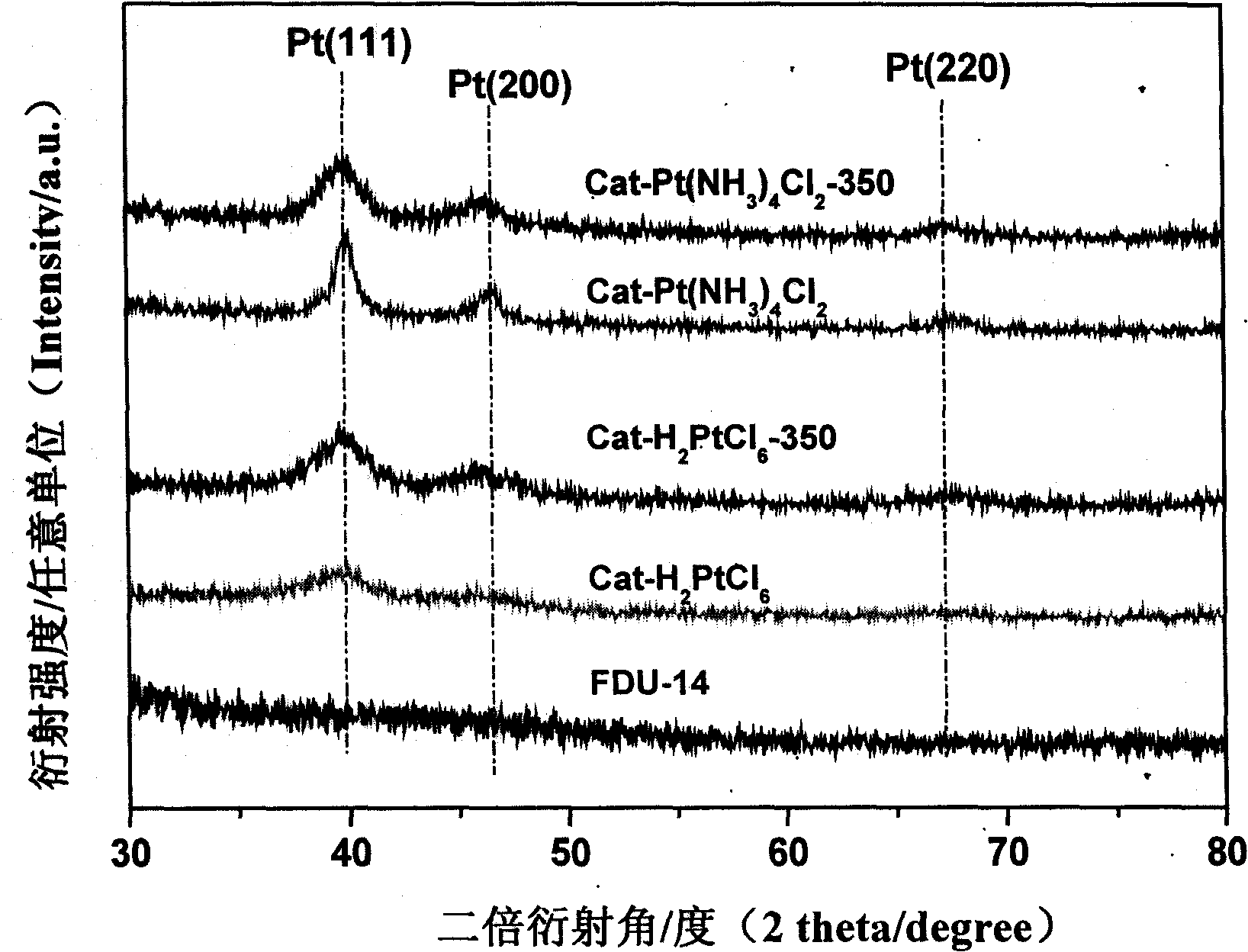
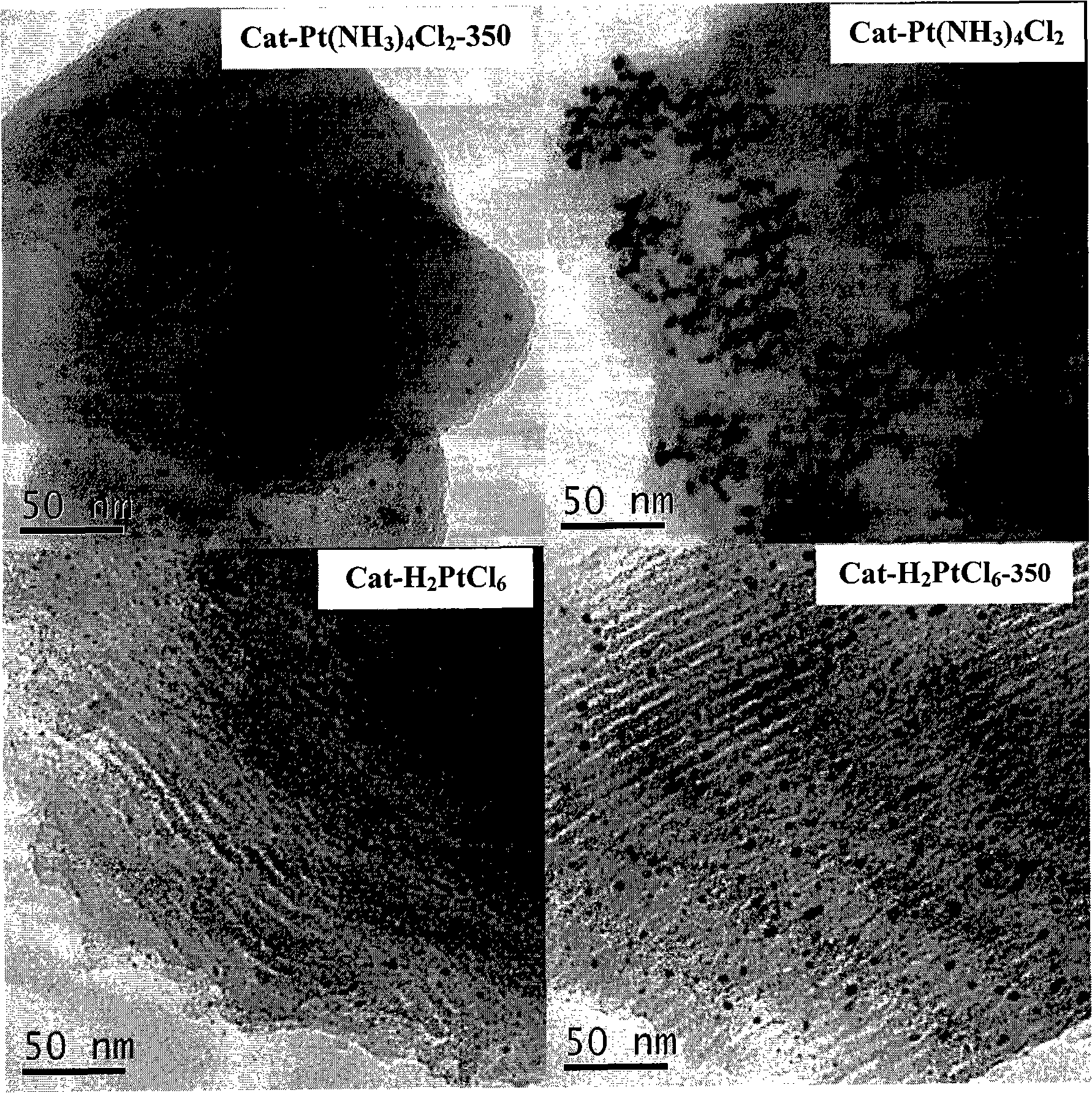
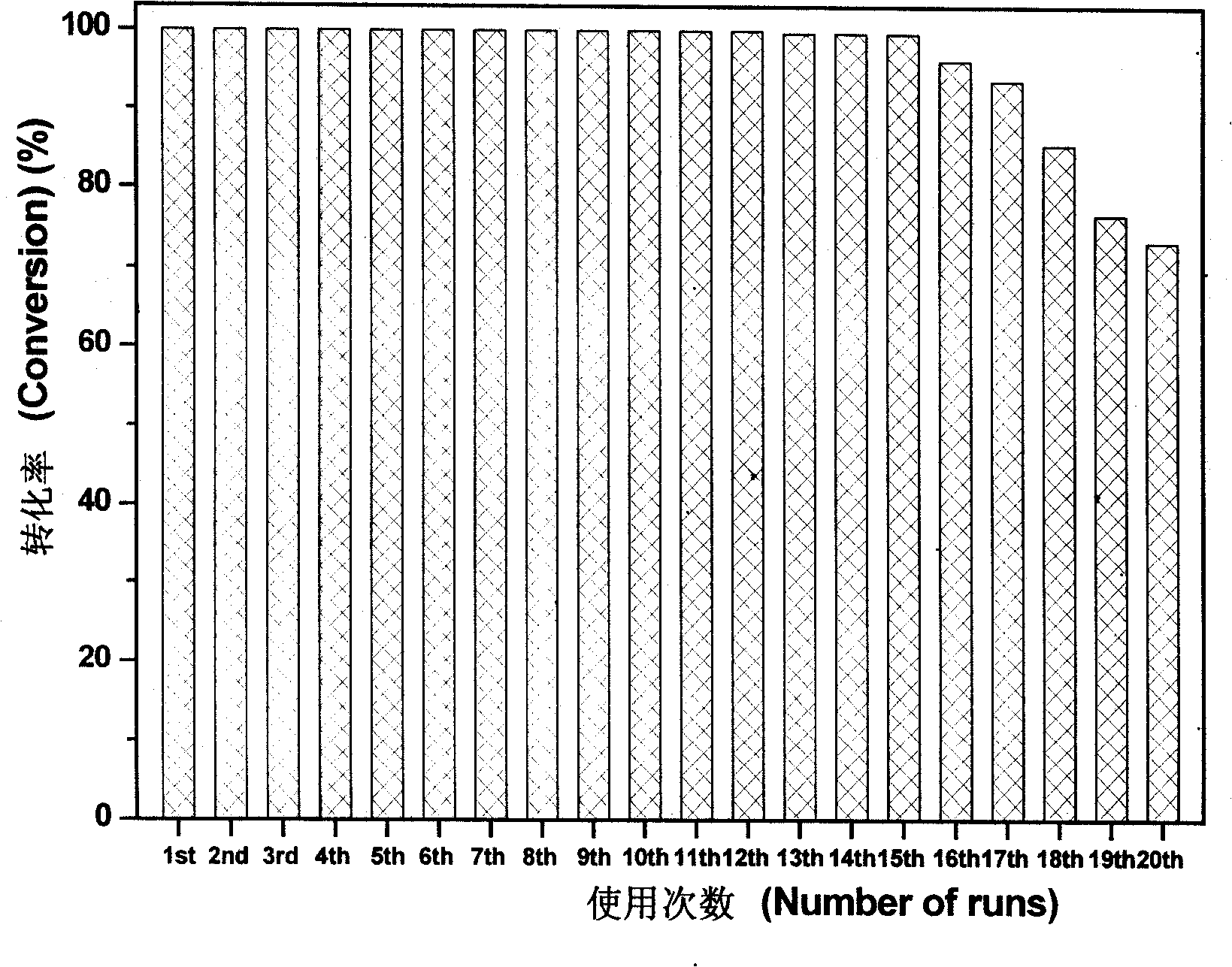
The invention relates to a heterogeneous catalyst used in an asymmetric catalytic hydrogenation reaction of Alpha-keto ester, and the preparation as well as the application thereof, and belongs to the technology field of a chemical catalyst and synthesis. The expression of the catalyst is Pt / FDU-14; when in preparation, platinic chloride or Tetrammine platinum (2) Chloride is used as an active component precursor, and FDU-14 is used as a carrier; part of catalyst precursor is vacuum-roasted in a muffle furnace after insuccation and torrefaction, and finally the catalyst precursor is deoxygenized in sodium formate water solution to prepare the heterogeneous catalyst, wherein, the dispersion degree of active component platinum on the surface of the FDU-14 is 0.14 to 0.35, the loading of metallic platinum is 5.0 percent, and the mean particle size of platinum particles ranges from 3.2 to 8.0nm. When the heterogeneous catalyst is used in the asymmetric catalytic hydrogenation reaction of ethyl pyruvate under the room temperature and the medium hydrogen pressure, high activity and optical selectivity more than 70 percent are obtained in acetic acid solution, and the heterogeneous catalyst can be repeatedly used for more than 15 times.
Owner:EAST CHINA NORMAL UNIV
Chiral alpha-amino acid derivative and preparation method thereof
ActiveCN107325025ASimple structureShort synthetic routeCarbamic acid derivatives preparationOrganic compound preparationSolventDiamine
The invention discloses a chiral alpha-amino acid derivative and a preparation method thereof. The structural formula of the chiral alpha-amino acid derivative disclosed by the invention is of formula I shown in the specification. The preparation method of the derivative comprises the following steps: mixing a carbonyl compound with a N,O-acetal mixture, a chiral primary tert-diamine organic small molecule catalyst, strong acid and weak acid, and performing a reaction, thereby obtaining the chiral alpha-amino acid derivative. The chiral alpha-amino acid derivative comprises aldehyde and / or ketone. The chiral alpha-amino acid derivative disclosed by the invention is prepared under catalysis of the chiral primary tert-diamine organic small molecule catalyst of a simple structure via one-step synthesis without solvent, thus being simple and efficient.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Thermal sprayed coating anti-reflection protective layer applicable to selective absorption of medium and high-temperature solar energy and preparation method of thermal sprayed coating anti-reflection protective layer
InactiveCN108122996AGood thermal stabilityImprove surface conditionFinal product manufactureLiquid/solution decomposition chemical coatingThermal sprayingMulti effect
The invention belongs to the field of solar energy selective absorption anti-reflection materials, and particularly relates to a thermal sprayed coating anti-reflection protective layer applicable toselective absorption of medium and high-temperature solar energy and a preparation method of the thermal sprayed coating anti-reflection protective layer. The thermal sprayed coating anti-reflection protective layer consists of an inner layer and an outer layer which are compounded, wherein the inner layer is prepared from 40 weight percent of a high-absorption Co3O4-CoAl2O4 thin film, and the outer layer is prepared from 20 weight percent of low-absorption Co3O4-CoAl2O4. The thermal sprayed coating anti-reflection protective layer prepared by the preparation method disclosed by the inventionnot only has excellent optical selectivity, but also can relieve the stress action of the coating in a thermal treatment process, reduce the porosity of a thermal sprayed coating and increase an absorption-emission ratio of the coating, is relatively high in thermal stability at high temperature, also achieves protection, connection and anti-reflection effects, and is compact in structure and stable in performance.
Owner:WUHAN UNIV OF TECH
Esterase, its DNA, its overexpression and production of optically active aryl propionic acids using the same
InactiveUS7223582B2Good optical selectivityOptical selectivitySugar derivativesBacteriaOptically activePhotochemistry
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Rhodococcus sp.D12 and its culture method and application
InactiveCN1128874COptimize culture conditionsHigh yieldBacteriaHydrolasesNitrogen sourceCarboxylic acid
A Rhodococcus sp.D12 is obtained by screening from soil, separating and purifying. Its optimized culture condition including kind of inductor, nitrogen source, carbon source, inducing period and dosage of inductor is also disclosed. Said strain can be used to catalytically hydrolyzing the nitrile compound into carboxylic acid and amide or their mixture.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Preparation method for chiral intermediate for use in statins
ActiveUS20170190684A1Low costLow yieldOrganic compound preparationCarboxylic acid esters preparationAcetic acidChloroacetic acids
The present invention relates to a preparation method for a chiral intermediate for use in statins, acquired with chloroacetic acid and benzyl alcohol as starting materials via a series of reactions, namely etherification, condensation, substitution, and asymmetric reduction. The preparation method provided in the present invention has a novel route of synthesis, allows an intermediate compound to be introduced conveniently into the chiral center of a glycol via enzyme reduction, and not only is low in costs, but also is reliable in quality. The route of synthesis provided in the present invention uses raw materials of low costs, has an easy to operate process, and provides a final product of great purity and high yield.
Owner:ASYMCHEM LAB TIANJIN +4
New preparation process of tasimeltion intermediate
The invention provides a novel preparation technology of a tasimelteon intermediate namely (1R,2R)-(2,3-dihydrobenzofuran-4-yl)cyclopropyl formic acid. The technology comprises the following steps: (1) carrying out reactions between 2,3-dihydrobenzofuran-4-formaldehyde and malonic acid in the presence of piperidine to generate 2,3-dihydrobenzofuran-4-acrylic acid; (2) converting 2,3-dihydrobenzofuran-4-acrylic acid into acyl chloride in the presence of thionyl chloride, then reacting the acyl chloride with L-menthol to generate 2,3-dihydrobenzofuran-4-acrylic acid L-menthol; (3) carrying out reactions between 2,3-dihydrobenzofuran-4-acrylic acid L-menthol and dimethyl sulfur oxide in the presence of sodium hydroxide to generate (1R,2R)-(2,3-dihydrobenzofuran-4-yl)cyclopropyl formic acid L-menthol; (4) hydrolyzing the (1R,2R)-(2,3-dihydrobenzofuran-4-yl)cyclopropyl formic acid L-menthol in the action of a sodium hydroxide water solution so as to obtain the (1R,2R)-(2,3-dihydrobenzofuran-4-yl)cyclopropyl formic acid. The novel preparation technology is developed on the basis of the conventional technologies, and has the advantages of easily-available raw materials, low cost, simple operation, and suitability for industrial massive production.
Owner:苏州莱克施德药业有限公司
A thermal spray coating anti-reflection protective layer suitable for selective absorption of medium and high temperature solar energy and its preparation method
InactiveCN108122996BHigh affinityImprove bindingFinal product manufactureLiquid/solution decomposition chemical coatingPorosityThermal spraying
The invention belongs to the field of solar energy selective absorption anti-reflection materials, and particularly relates to a thermal sprayed coating anti-reflection protective layer applicable toselective absorption of medium and high-temperature solar energy and a preparation method of the thermal sprayed coating anti-reflection protective layer. The thermal sprayed coating anti-reflection protective layer consists of an inner layer and an outer layer which are compounded, wherein the inner layer is prepared from 40 weight percent of a high-absorption Co3O4-CoAl2O4 thin film, and the outer layer is prepared from 20 weight percent of low-absorption Co3O4-CoAl2O4. The thermal sprayed coating anti-reflection protective layer prepared by the preparation method disclosed by the inventionnot only has excellent optical selectivity, but also can relieve the stress action of the coating in a thermal treatment process, reduce the porosity of a thermal sprayed coating and increase an absorption-emission ratio of the coating, is relatively high in thermal stability at high temperature, also achieves protection, connection and anti-reflection effects, and is compact in structure and stable in performance.
Owner:WUHAN UNIV OF TECH
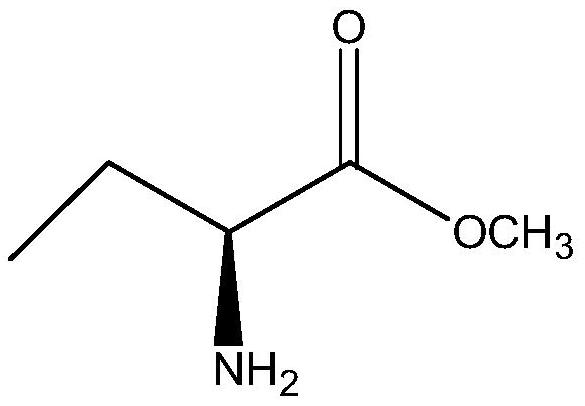
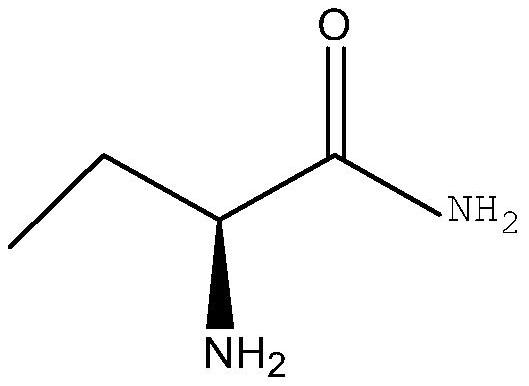
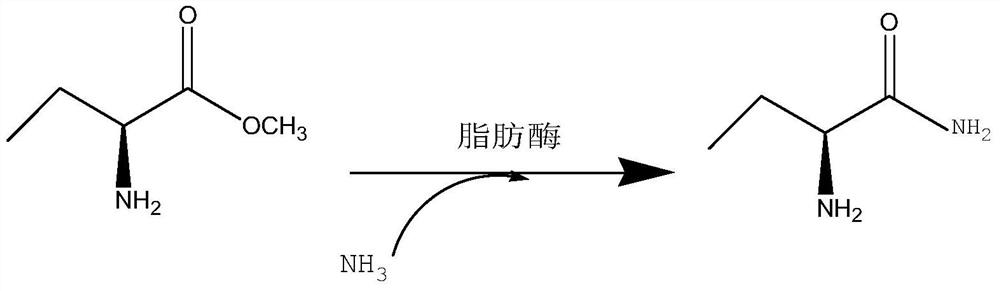
A kind of method for enzymatic synthesis of (s)-2-aminobutanamide
The invention relates to a method for enzymatically synthesizing (S)-2-aminobutanamide, and belongs to the technical field of pharmaceutical intermediate synthesis. In order to solve the existing problem of adopting chemical synthesis method, a method for enzymatic synthesis of (S)-2-aminobutanamide is provided, the method comprises in the presence of an amino donor, under the action of a catalytic amount of lipase, The substrate (S)-2-aminobutyric acid methyl ester is subjected to catalytic aminolysis to obtain the corresponding product (S)-2-aminobutyramide; the gene sequence of the lipase is shown in SEQ ID NO.1. The present invention has high stereoselectivity and specificity, forms a product of S-type chiral configuration, and has the effects of high chiral purity and high ee value of the product.
Owner:江苏八巨药业有限公司
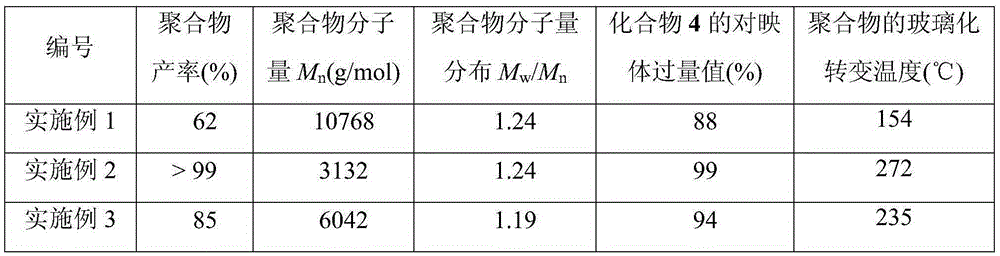
Polycarbonate with optical activity
The invention relates to polycarbonate with optical activity and a preparation method thereof, belonging to the technical field of polymer synthesis. A repetitive unit of the polycarbonate is shown in the description, wherein R1-R3 are -H or alkyl. The preparation method of the polycarbonate comprises the following steps: dispersing an epoxide and a catalyst into an organic solvent, and performing a carbon dioxide bubbling reaction for 30-40h, thus obtaining the polycarbonate; the epoxide is shown in the description. The prepared polycarbonate has optical activity, a number-average molar mass of more than 3000, and a vitrification transformation temperature being more than 150 DEG C, maximally 272 DEG C, and the polycarbonate can be used as a high temperature-resistant polymer material and be applied to fields with harsh work environments. The preparation method can be implemented under room temperature, the pressure requirement on carbon dioxide is very low, reaction conditions are mild, the optical selectivity is high, and a prepared product has extremely high optical purity and wide industrial promotion value.
Owner:郑州裕昌建筑节能科技有限公司
Shakubiqu intermediate, Shakubiqu intermediate and preparation method of Shakubiqu
ActiveCN105924355BImprove qualityHigh yieldOrganic compound preparationCarboxylic acid esters preparationChemical reactionOrganic synthesis
The invention discloses a sacubitril intermediate and a preparation method of the sacubitril intermediate and sacubitril, and belongs to the technical field of organic synthesis of drugs. On one hand, the invention discloses a novel compound-sacubitril intermediate compound B and a preparation method thereof, and on the other hand, the invention discloses a novel preparation method of the sacubitril. The sacubitril intermediate and the preparation method of the sacubitril intermediate and the sacubitril have the following advantages that the synthesizing route is short, the product can be prepared only through four steps of chemical reactions, and the steps of existing patent routes all exceed nine steps; the dicarbonyl compound is prepared through Reformasky condensation supplied by the method, and the cost is low; a first chiral center is introduced by fully utilizing a chiral compound L-(S)-ethyl lactate which is low in cost and easy to obtain in the natural world, and the cost is low; a second chiral center is constructed through a biological enzyme catalysis technique, the optical selectivity reaches up to 99.9%, the quality is good, and the cost is low. By means of the technological means, the total yield of the prepared sacubitril is increased, and the quality of the sacubitril is improved.
Owner:ZHEJIANG HONGYUAN PHARMA
Preparation method of ibrutinib
InactiveCN113200987AEasy to prepareGood optical selectivityOrganic chemistryBiochemical engineeringSuzuki reaction
The invention relates to a preparation method of ibrutinib and an intermediate thereof, belonging to the field of medicinal chemistry. According to the preparation method, the ibrutinib can be obtained through condensation, condensation, substitution, substitution and Suzuki reaction by taking a low-cost material flow as a starting material; and the method is low in cost, free of light delay reaction, high in yield, good in selectivity, short in route, few in generated three wastes and suitable for industrial large-scale production.
Owner:湖南华腾制药有限公司
Enzymatic Preparation of Chiral 3-Hydroxytetrahydrofuran and Alcohol Dehydrogenase Mutants
ActiveCN106520714BReduce pollutionImprove efficiencyMicroorganism based processesOxidoreductasesAlcohol3-Hydroxytetrahydrofuran
The invention relates to a process for enzymatic preparation of chiral 3-hydroxytetrahydrofuran and an alcohol dehydrogenase mutant. The process comprises the a step of, with 3-ketotetrahydrofuran as a substrate, subjecting the substrate, the alcohol dehydrogenase mutant, a cofactor and a cofactor regeneration system to a biological catalysis reaction in a buffer system so as to produce chiral 3-hydroxytetrahydrofuran, wherein for alcohol dehydrogenase used by the alcohol dehydrogenase mutant, the wild gene sequence of alcohol dehydrogenase is derived from thermophilic bacteria, the base sequence is as shown in SEQ ID No. 1, and the amino acid sequence is as shown in SEQ ID No. 2. Compared with chemical methods, the process using the alcohol dehydrogenase mutant for preparation of chiral 3-hydroxytetrahydrofuran has the advantages of small environmental pollution, high efficiency, high optical selectivity; and compared with enzyme resolution methods, the process provided by the invention has higher purity and conversion rate and has ee value and conversion rate of both 99%.
Owner:洛阳华荣生物技术有限公司
Chiral organic selenium sulfur catalyst and its preparation method and application in asymmetric reaction
InactiveCN105665011BWide range of usesGood catalyticOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSulfurOrganic reaction
The invention discloses a chiral organic selenium-sulfur catalyst and its preparation method and use in an asymmetric reaction. The chiral organic selenium-sulfur catalyst is prepared from commercial and low price chiral amino-indanol through high efficiency multi-step synthesis. The preparation method has simple processes and is convenient for operation. The chiral organic selenium-sulfur catalyst based on an organic indene skeleton has a wide application range, good catalysis effects in many organic reactions and excellent enantiomer selectivity. The chiral organic selenium-sulfur catalyst has excellent optical selectivity in an asymmetric trifluoromethylthioesterification reaction, opens the ancient river of an alkene asymmetric trifluoromethylthioesterification reaction and has high catalysis efficiency and good enantioselectivity.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com